Abstract
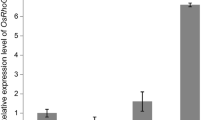
A late-flowering transgenic radish has been produced by the expression of an antisense GIGANTEA (GI) gene fragment using a floral-dip method. Twenty-five plants were dipped into a suspension of Agrobacterium carrying a 2.5 kb antisense GI gene fragment from Arabidopsis, along with the gusA and bar reporter genes, all under the control of a CaMV 35S promoter. From a total of 1462 seeds harvested from these floral-dipped plants, 16 Basta-resistant T1 plants were found to have GUS activity (transformation efficiency of 1.1%). Southern analysis confirmed the integration of one or two copies of the gusA gene in these herbicide-resistant plants. Expression of the GI gene in T1 plants was much reduced compared to both wildtype plants and plants transformed with pCAMBIA3301 (positive control). In the progenies of eleven T1 plants analysed (T2 generation), all lines showed a significant delay in both bolting and flowering times compared to wildtype and positive control plants, and that, the level of GI transcript was inversely proportional to the time of bolting and flowering. At a maximum, bolting and flowering times were delayed by 17 and 18 days respectively, compared to wildtype plants (in positive control plants, the delay was 23 and 26 days, respectively). Ten of the 11 lines exhibited a significant reduction in plant height compared to wildtype and positive control plants. This study provides evidence that down-regulation of the GI gene by co-suppression could delay bolting in a cold-sensitive long-day (LD) plant. Production of late-flowering germplasms of radish may allow this important crop to be cultivated over an extended period and also provide further food to the famine countries of S/E Asia.
Similar content being viewed by others
References
Araki T and Komeda Y (1993) Analysis of the role of the late-flowering locus, I, in the flowering of Arabidopsis thaliana. Plant J 3: 231–239.
Bernier G, Havelange A, Houssa C, Petitjean A and Lejeune P (1993) Physiological signals that induce flowering. Plant Cell 5: 1147–1155.
Burn JE, Bagnall DJ, Metzger JD, Dennis ES and Peacock WJ (1993) DNA methylation, vernalization and the initiation of flowering. Proc Natl Acad Sci USA 90: 287–291.
Chomczynski P and Mackey K (1995) Modification of the TRI ReagentTM procedure for isolation of RNA from polysaccharide–and proteoglycan–rich sources. Biotechniq 19: 942–945.
Clough SJ and Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743.
Coetzer C, Corsini D, Love S, Pavek J and Tumer N (2001) Control of enzymatic browning in potato (Solanum tuberosum L.) by sense and antisense RNA from tomato polyphenol oxidase. J Agric Food Chem 49: 652–657.
Curtis IS and Nam HG (2001) Transgenic radish (Raphanus sativus L. var. longipinnatus Bailey) by floral-dip method–plant development and surfactant are important in optimizing transformation efficiency. Trans Res 10: 363–371.
Curtis IS, Power JB, Hedden P, Phillips A, Lowe KC, Ward DA et al. (2000) Transformation and characterization of transgenic plants of Solanum dulcamara L.–incidence of transgene silencing. Ann Bot 86: 63–71.
Dellaporta SL, Wood J and Hicks IB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1: 19–21.
Desfeux C, Clough SJ and Bent AF (2000) Female reproductive tissues are the primary target of Agrobacterium-mediated transformation by the Arabidopsis floral-dip method. Plant Physiol 123: 895–904.
Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B et al. (1999) GIGANTEA: a circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J 18: 4679–4688.
Huq E, Tepperman JM and Quail PH (2000) GIGANTEA is a nuclear protein involved in phytochrome signaling in Arabidopsis. Proc Natl Acad Sci USA 97: 9789–9794.
Koornneef M, Hanhart CJ and van der Veen JH (1991) A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet 229: 57–66.
Koornneef M, van Eden J, Hanhart CJ, Stam P, Braaksma FJ and Feenstra WJ (1983) Linkage map of Arabidopsis thaliana. J Hered 74: 265–272.
Lazo GR, Stein PA and Ludwig RA (1991) A DNA transformationcompetent Arabidopsis genomic library in Agrobacterium. Bio/Technol 9: 963–967.
Lee S-S (1987) Bolting in radish. In: Asian and Pacific Council (eds), Improved Vegetable Production in Asia. (pp. 60-70) Food & Fertilizer Technology Center, Book series no. 36, Taipei, Taiwan, Republic of China.
Meyer P, Linn F, Heidmann I, Meyer H, Niedenhof I and Saedler H (1992) Endogenous and environmental factors influence 35S promoter methylation of a maize A1 gene construct in transgenic petunia and its colour phenotype. Mol Gen Genet 231: 345–352.
Park DH, Somers DE, Kim YS, Choy YH, Lim HK, Soh MS et al. (1999) Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science 285: 1579–1582.
Redei GP (1962) Supervital mutants of Arabidopsis. Genetics 47: 443–460.
Reeves PH and Coupland G (2000) Response of plant development to environment: control of flowering by daylength and temperature. Curr Opin Plant Biol 3: 37–42.
Sheldon CC, Finnegan EJ, Rouse DT, Tadege M, Bagnall DJ, Helliwell CA, Peacock WJ and Dennis ES (2000) The control of flowering by vernalization. Curr Opin Plant Biol 3: 418–422.
Smith CJS, Watson CF, Morris PC, Bird CR, Seymour GB, Gray JE et al. (1990) Inheritance and effect on ripening of antisense polygalacturonase genes in transgenic tomatoes. Plant Mol Biol 14: 369–379.
Trieu AT, Burleigh SH, Kardailsky IV, Maldonado-Mendoza IE, Versaw WK, Blaylock LA et al. (2000) Transformation of Medicago truncatula via infiltration of seedlings or flowering plants with Agrobacterium. Plant J 22: 531–541.
Ye GN, Stone D, Pang SZ, Creely W, Gonzalez K and Hinchee M (1999) Arabidopsis ovule is the target for Agrobacterium in planta vacuum infiltration transformation. Plant J 19: 249–257.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Curtis, I.S., Nam, H.G., Yun, J.Y. et al. Expression of an antisense GIGANTEA (GI) gene fragment in transgenic radish causes delayed bolting and flowering. Transgenic Res 11, 249–256 (2002). https://doi.org/10.1023/A:1015655606996
Issue Date:
DOI: https://doi.org/10.1023/A:1015655606996