Abstract
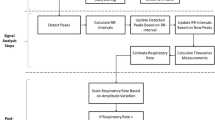
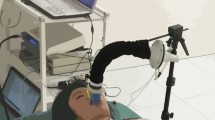
Objective.Photoplethysmography (PPG) is a non-invasive optical technique that measures variations in skin blood volume and perfusion. The PPG signal contains components that are synchronous with respiratory and cardiacrhythms. We undertook this study to evaluate PPG for monitoring patients' respiratory rate in the postoperative care unit, using a new prototype device. We compared it with the established technique, transthoracic impedance (TTI). Methods.PPG signals from 16 patients(ASA classes 1–2, mean age 43 years) who were recovering from general anaesthesia after routine operations were recorded continuously for 60minutes/patient. The respiratory synchronous part of the PPG signal was extracted by using a band pass filter. Detection of breaths in the filtered PPG signals was done both visually and by using an automated algorithm. In both procedures, the detected breaths were compared with the breaths detected in the TTI reference. Results.A total of 10.661 breaths were recorded, and the mean ± SD respiratory rate was 12.3 ± 3.5breaths/minute. When compared with TTI, the rates of false positive and false negative breaths detected by PPG (visual procedure) were 4.6 ±4.5% and 5.8 ± 6.5%, respectively. When using the algorithm for breath detection from PPG, the rates of false positive andfalse negative breaths were 11.1 ± 9.7% and 3.7 ±3.8%, respectively, when compared to TTI. Lower respiratory rates increased the occurrence of false-positive breaths that were detected by the PPG using visual identification (p< 0.05). The same tendency was seen with the automated PPG procedure (p< 0.10). Conclusions.Our results indicate that PPG has the potential to be useful for monitoring respiratory rate in the postoperative period.
Similar content being viewed by others
REFERENCES
Jones JG, Sapsford DJ, Wheatley RG. Postoperative hypoxaemia: mechanisms and time course. Anaesthesia 1990; 45: 566–573
Nimmo AF, Drummond GB. Respiratory mechanics after abdominal surgery measured with continuous analysis of pressure, Flow and volume signals. Br J Anaesth 1996; 77: 317–326
Gill NP, Wright B, Reilly CS. Relationship between hypoxaemic and cardiac ischaemic events in the perioperative period. Br J Anaesth 1992; 68: 471–473
Rosenberg J, Kehlet H. Postoperative mental confusion — association with postoperative hypoxemia. Surgery 1993; 114: 76–81
Drummond GB, Nimmo AF, Elton RA. Thoracic impedance used for measuring chest wall movement in post-operative patients. Br J Anaesth 1996; 77: 327–332
Cyna AM, Kulkarni V, Tunstall ME, et al. Aura: A new respiratory monitor and apnoea alarm for spontaneously breathing patients. Br J Anaesth 1991; 67: 341–345
Gordh T, Rawal N, Stroöm S, Hoök B. Respiratory monitoring during postoperative analgesia. J Clin Monit 1995; 11: 365–372
Cohn MA, Rao ASV, Broudy M, et al. The respiratory inductive plethysmograph: A non-invasive monitor of respiration. Bull Eur Physiopathol Respir 1982; 18: 643–658
Anderson W, Brock-Utne AJ, Brock-Utne JG, Brodsky JB. Evaluation of a respiratory rate monitor in post-surgical patients. J Clin Anesth 1992; 4: 289–291
Tremper KK, Barker SJ. Pulse oximetry. Anesthesiology 1989; 70: 98–108
Moöller JT, Johannessen NW, Espersen K, et al. Randomized evaluation of pulse oximetry in 20,802 patients: II. Anesthesiology 1993; 78: 445–453
Dumas C, Wahr JA, Tremper KK. Clinical evaluation of a prototype motion artifact resistant pulse oximeter in the recovery room. Anesth Analg 1996; 83: 269–272
Standards of the American Society of Anesthesiologists. Standards for postanesthesia care. Last amended on October 19, 1994; http://www.asahg.org
Kamal AAR, Harness JB, Irving G, Mearns AJ. Skin photoplethysmography — a review. Comp Methods Prog Biomed 1989; 28: 257–269
Nilsson L, Johansson A, Svanerudh J, Kalman S. Is the respiratory component of the photoplethysmographic signal of venous origin? Med Biol Eng Comput 1999; 37 (Suppl 2): 912–913
Ugnell H. The respiratory synchronous photoplethys-mographic signal. Its dependence on light wavelength and sample volume. Med Biol Eng Comput 1996; 34 (Suppl 1, pt1): 275–276
Dorlas JC, Nijboer JA. Photo-electric plethysmography as a monitoring device in anaesthesia. Br J Anaesth 1985; 57: 524–530
Johansson A, Öberg PÅ, Sedin G. Monitoring of heart and respiratory rates in newborn infants using a new photoplethysmographic technique. J Clin Monit 1999; 15: 461–467
Johansson A, Nilsson L, Kalman S, Öberg PÅ. Monitoring of Respiratory Rates using Photoplethysmography, 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Hong Kong, 1998, Report No. 3226
Lindberg L-G, Ugnell H, Öberg PÅ. Monitoring of respiratory and heart rates using a fibre-optic sensor. Med Biol Eng Comput 1992; 30: 533–537
Larsen PD, Harty M, Thiruchelvam M, Galletly DC. Spectral analysis of AC and DC components of the pulse photoplethysmograph at rest and during induction of anaesthesia. Int J Clin Monit Comput 1997; 14: 89–95
Johansson A, Öberg PÅ Estimation of respiratory volumes from the photoplethysmographic signal. Part 1: Experimental results. Med Biol Eng Comput 1999; 37: 42–47
Galletly DC, Williams TB, Robinson BJ. Periodic cardiovascular and ventilatory activity during midazolam sedation. Br J Anaesth 1996; 76: 503–507
Hayes MJ, Smith PR. Artifact reduction in photoplethysmography. Applied Optics 1998; 37: 7437–7446
Tur E, Tur M, Maibach HI, Guy RH. Basal perfusion of the cutaneous microcirculation: Measurements as a function of anatomic position. J Invest Dermatol 1983; 81: 442–446
Ziege S, Schmid-Schoönbein H, Grebe R, Martin E. Long-term registration of cutaneous microcirculation during general anesthesia. Int J Microcirc Clin Exp 1997; 17: 385–394
Hall JM, Lampotang S, Thoman J, et al. A continuous respiratory rate monitor derived from the optoplethys-mogram of a pulse oximeter: Clinical evaluation. Anesthesiology 1998; 89 (3A): A 971
Wiklund L, Hoök B, Staåhl K, Jordeby-Joönsson A. Post-anesthesia monitoring revisited: frequency of true and false alarms from different monitoring devices. J Clin Anesth 1994; 6: 182–188
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nilsson, L., Johansson, A. & Kalman, S. Monitoring of respiratory rate in postoperative care using a new photoplethysmographic technique. J Clin Monit Comput 16, 309–315 (2000). https://doi.org/10.1023/A:1011424732717
Issue Date:
DOI: https://doi.org/10.1023/A:1011424732717