Abstract
Purpose. A new mucus-secreting in vitro drug absorption model based on monolayers of goblet-cell like sub-clones of the human colon carcinoma cell line HT29 obtained by methotrexate (MTX) treatment was investigated.
Methods. Twelve sub-clones were isolated and characterized by light microscopy (LM), transelectron microscopy (TEM), confocal laser scanning microscopy (CLSM), transepithelial electrical resistance (TEER) and the transport of a paracellular marker FITC-Dextran (Mw 4400) (FD-4).
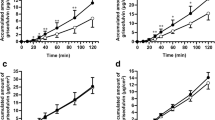
Results. Significant differences of microscopical appearance, TEER-values and permeability of FD-4 between the sub-clones were evident. However, two of them, namely MTX-D1 and MTX-E12, formed tight confluent monolayers with a thick mucus-layer on the apical surface. They were used to compare the apparent permeability coefficient (Papp) of a series of lipophilic drugs, which should be affected by the mucus-layer, namely barbiturates (barbituric acid, barbital, phenobarbital, methylphenobarbital and heptabarbital) and testosterone, as a reference, to mucus-free Caco-2 cells. The permeability of drugs with a partition coefficient (log P) > 1 was decreased in the mucus-producing cell lines. Testosterone, the most lipophilic compound, showed a decrease of up to 43%.
Conclusions. We demonstrated that the mucus layer is a significant barrier to drug absorption for lipophilic drugs. In conclusion, our model may serve as a suitable in-vitro cell culture model to study the influence of the mucus layer on drug diffusion.
Similar content being viewed by others
REFERENCES
J. Karlsson and P. Artursson. A new diffusion chamber system for the determination of drug permeability coefficients across the human intestinal epithelium that are independent of the unstirred water layer. Biochim. Biophys. Acta. 1111:204-210 (1992).
A. Adson, P. S. Burton, T. J. Raub, C. L. Barsuhn, K. L. Audus, and N. F. Ho. Passive diffusion of weak organic electrolytes across Caco-2 cell monolayers: Uncoupling the contribution of hydrodynamics, transcellular and paracellular barriers. J. Pharm. Sci. 84:1197-1204 (1995).
D. Winne and W. Verheyen. Diffusion coefficient in native mucus gel of rat small intestine. J. Pharm. Pharmacol. 42:517-519 (1990).
F. Nimmerfall and J. Rosenthaler. Significance of the goblet-cell mucin layer, the outermost luminal barrier to passage through the gut wall. Biochem. Biophys. Res. Comm. 94:960-966 (1980).
A. Wikman-Larhed, P. Artursson, J. Grasjo, and E. Bjork. Diffusion of drugs in native and purified gastrointestinal mucus. J. Pharm. Sci. 86:660-665 (1997).
J. Karlsson, A. Wikman, and P. Artursson. The mucus layer as a barrier to drug absorption in monolayers of human intestinal epithelial HT29-H goblet cells. Int. J. Pharm. 99:209-218 (1993).
M. Jackson. Drug transport across the gastrointestinal epithelia. In L. R. Johnson (ed.), Physiology of the Gastrointestinal Tract, Raven Press, New York, 1987 pp. 1597-1621.
P. Artursson and R. T. Borchardt. Intestinal drug absorption and metabolism in cell cultures: Caco-2 and beyond. Pharm. Res. 14:1655-1658 (1997).
A. Zweibaum, M. Pinto, G. Chevalier, E. Dussaulx, N. Triadou, B. Lacroix, K. Haffen, J.-L. Brun, and M. Rousset. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT29 selected for growth in sugar-free medium and its inhibitions by glucose. J. Cell Physiol. 122:21-29 (1985).
C. Huet, C. Sahuquillo-Merino, E. Coudrier, and D. Louvard. Absorptive and mucus-secreting sub-clones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J. Cell Biol. 105:345-357 (1987).
T. Lesuffleur, A. Barbat, E. Dussaulx, and A. Zweibaum. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 50:6334-6343 (1990).
A. Wikman, J. Karlsson, I. Carlstedt, and P. Artursson. A drug absorption model based on the mucus layer producing human intestinal goblet cell line HT29-H. Pharm. Res. 10:843-852 (1993).
N. G. Schipper, K. M. Varum, P. Stenberg, G. Ocklind, H. Lennernäs, and P. Artursson. Chitosans as absorption enhancers of poorly absorbable drugs. 3: Influence of mucus on absorption enhancement. Eur. J. Pharm. Sci. 8:335-343 (1999).
E. Walter and T. Kissel. Heterogeneity in the human intestinal cell line Caco-2 leads to differences in transepithelial transport. Eur. J. Pharm. Sci. 3:215-230 (1995).
E. K. Anderberg, T. Lindmark, and P. Artursson. Sodium caprate elicits dilatations in human intestinal tight junctions and enhances drug absorption by the paracellular route. Pharm. Res. 10:857-864 (1993).
S. Wagner, M. L. Enss, M. Cornberg, H. Mix, S. Schumann, G. Kirchner, J. Jahne, M. P. Manns, and W. Beil. Morphological and molecular characterization of human gastric mucus cells in long-term primary culture. Pflugers Arch. 436:871-881 (1998).
S. Yee. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man-fact or myth. Pharm. Res. 14:763-766 (1997).
M. D. Burdick, A. Harris, C. J. Reid, T. Iwamura, and M. A. Hollingsworth. Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J. Biol. Chem. 272:24198-24202 (1997).
M. P. Buisine, L. Devisme, T. C. Savidge, C. Gespach, B. Gosselin, N. Porchet, and J. P. Aubert. Mucin gene expression in human embryonic and fetal intestine. Gut 43:519-524 (1998).
A. Biragyn, S. Arkins, and K. W. Kelley. Riboprobe expression cassettes for measuring IGF-I, beta-actin and glyceraldehyde 3-phosphate dehydrogenase transcripts. J. Immunol. Methods 168:235-244 (1994).
W. Kamm, A. Jonczyk, T. Jung, G. Luckenbach, P. Raddatz, and T. Kissel. Evaluation of absorption enhancement for a potent cyclopeptidic alpha(nu)beta(3)-antagonist in a human intestinal cell line (Caco-2). Eur. J. Pharm. Sci. 10:205-214 (2000).
A. Wikman-Larhed, P. Artursson, and E. Björk. The influence of intestinal mucus components on the diffusion of drugs. Pharm. Res. 15:66-71 (1998).
J. L. Madara and J. S. Trier. Structure and permeability of goblet cell tight junctions in rat small intestine. J. Membr. Biol. 66:145-157 (1982).
T. Lesuffleur, A. Barbat, C. Luccioni, J. Beaumatin, M. Clair, A. Kornowski, E. Dussaulx, B. Dutrillaux, and A. Zweibaum. Dihydrofolate reductase gene amplification-associated shift of differentiation in methotrexate-adapted HT-29 cells. J. Cell Biol. 115: 1409-1418 (1991).
I. Matthes, F. Nimmerfall, and H. Sucker. Mucus models for investigation of intestinal absorption mechanisms. 1. Validation and optimization of the model. Pharmazie. 47:505-515 (1992).
B. Sandzen, H. Blom, and S. Dahlgren. Gastric mucus gel layer thickness measured by direct light microscopy. An experimental study in the rat. Scand. J. Gastroenterol. 23:1160-1164 (1988).
E. F. Hounsell and T. Feizi. Gastrointestinal mucins. Structures and antigenicities of their carbohydrate chains in health and disease. Med. Biol. 60:227-236 (1982).
T. Lesuffleur, N. Porchet, J. P. Aubert, D. Swallow, J. R. Gum, Y. S. Kim, F. X. Real, and A. Zweibaum. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J. Cell Sci. 106:771-783 (1993).
B. J. van Klinken, E. Oussoren, J. J. Weenink, G. J. Strous, H. A. Buller, J. Dekker, and A. W. Einerhand. The human intestinal cell lines Caco-2 and LS174T as models to study cell-type specific mucin expression. Glycoconj. J. 13:757-768 (1996).
R. D. Specian and M. G. Oliver. Functional biology of intestinal goblet cells. Am. J. Physiol. 260:C183-C193 (1991).
A. Wikman-Larhed and P. Artursson. Co-cultures of human intestinal goblet (HT29-H) and absorptive (Caco-2) cells for studies of drug and peptide absorption. Eur. J. Pharm. Sci. 3:171-183 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Behrens, I., Stenberg, P., Artursson, P. et al. Transport of Lipophilic Drug Molecules in a New Mucus-Secreting Cell Culture Model Based on HT29-MTX Cells. Pharm Res 18, 1138–1145 (2001). https://doi.org/10.1023/A:1010974909998
Issue Date:
DOI: https://doi.org/10.1023/A:1010974909998