Abstract
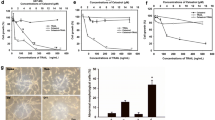
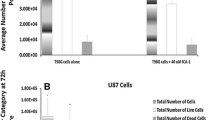
Most tumors, including gliomas, are resistant to tumor necrosis factor (TNF) cytotoxicity unless protein or RNA synthesis is inhibited. We investigated the effects of the combined use of TNF-α and cisplatin (CDDP) on cultured malignant glioma cells, T98G, U373MG, A172, and U87MG. All glioma cell lines were sensitive to treatment with CDDP but resistant to TNF-α during 24 h-incubation. The combined use of CDDP and TNF-α had synergistic effects on T98G and U87MG but not on U373MG and A172 cells. Sequential treatments showed that only pretreatment with CDDP for 2 h followed by TNF-α for 22 h was synergistic on cell cytotoxicity. Annexin V-flow cytometry and terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labeling assay showed that TNF-α can induce apoptosis in cells treated with CDDP. Although only sensitive cell lines express transcripts for p75 TNF receptor 2, changes in TNF receptors were not found to contribute to the susceptibility to TNF-α. The production of interleukin-6, a representative cytoprotective cytokine, from glioma cells stimulated by TNF-α was suppressed by the combined use of actinomycin D, but not CDDP. Our results indicate that CDDP can sensitize glioma cells to TNF-α-induced apoptosis by a mechanism other than blocking the cytoprotective protein production.
Similar content being viewed by others
References
Black PM: Brain tumors (second of two parts). N Engl J Med 324: 1555–1564, 1991
Wilson CB: Brain tumors. N Engl J Med 300: 1469–1471, 1979
Devaux BC, O'Fallon JR, Kelley PJ: Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. J Neurosurg 73: 767–775, 1993
Wakimoto H, Aoyagi M, Nakayama T, Nagashima G, Yamamoto S, Tamaki M, Hirakawa K: Prognostic significance of Ki-67 labeling indices obtained using MIB-1 monoclonal antibody in patients with supratentorial astrocytomas. Cancer 77: 373–380, 1996
Hall WA: Targeted toxin therapy for malignant astrocytoma. Neurosurgery 46: 544–551, 2000
Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, Long Z, Chiang Y, McGarrity GJ, Muul LM, Katz D, Blaese RM, Oldfield EH: Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med 3: 1354–1361, 1997
Chadha M, Capala J, Coderre JA, Elowitz EH, Iwai J, Joel DD, Liu HB, Wielopolski L, Chanana AD: Boron neutron-capture therapy (BNCT) for glioblastoma multiforme (GBM) using the epithermal neutron beam at the Brookhaven National Laboratory. Int J Radiat Oncol Biol Phys 40: 829–834, 1998
Hall WA, Djalilian HR, Sperduto PW, Cho KH, Gerbi BJ, Gibbons JP, Rohr M, Clark HB: Stereotactic radiosurgery for recurrent malignant gliomas. J Clin Oncol 13:1642–1648, 1995
Sugarman BJ, Aggarwal BB, Hass PE, Figari IS, Palladino MA Jr, Shepard HM: Recombinant human tumor necrosis factor-α: Effects on proliferation of normal and transformed cells in vitro. Science 230: 943–945, 1985
Tsujimoto M, Yip YK, VilČek J: Tumor necrosis factor: specific binding and internalization in sensitive and resistant cells. Proc Natl Acad Sci USA 82: 7626–7630, 1985
Ruggiero V, Latham K, Baglioni C: Cytostatic and cytotoxic activity of tumor necrosis factor on human cancer cells. J Immunol 138: 2711–2717, 1987
Larrick JW, Wright SC: Cytotoxic mechanism of tumor necrosis factor-?. FASEB J 4: 3215–3223, 1991
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B: An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA 72: 3666–3670, 1975
Watanabe N, Niitsu Y, Neda H, Sone H, Yamauchi N, Umetsu T, Urushizaki I: Antitumor effect of tumor necrosis factor against various primarily cultured human cancer cells. Jpn J Cancer Res 76: 1115–1119, 1985
Furman WL, Strother D, McClain K, Bell B, Leventhal B, Pratt CB: Phase I clinical trial of recombinant human tumor necrosis factor in children with refractory solid tumors: a Pediatric Oncology Group study. J Clin Oncol 11: 2205–2210, 1993
Lenk H, Tanneberger S, Müller U, Ebert J, Shiga T: Phase II clinical trial of high-dose recombinant human tumor necrosis factor. Cancer Chemother Pharmacol. 24: 391–392, 1989
Yoshida J, Wakabayashi T, Mizuno M, Sugita K, Yoshida T, Hori S, Mori T, Sato T, Karashima A, Kurisu K, Kiya K, Uozumi T: Clinical effect of intra-arterial tumor necrosis factor-α for malignant glioma. J Neurosurg 77: 78–83, 1992
Smith CA, Davis T, Anderson D, Solam L, Beckmann MP, Jerzy R, Dower SK, Cosman D, Goodwin RG: Areceptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science 248: 1019–1023, 1990
Loetscher H, Pan Y-CE, Lahm H-W, Gentz R, Brockhaus M, Tabuchi H, Lesslauer W: Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell 61: 351–359, 1990
Brockhaus M, Schoenfeld H-J, Schlaeger E-J, Hunziker W, Lesslauer W, Loetscher H: Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad SciUSA87: 3127–3131, 1990
Loetscher H, Schlaeger E-J, Lahm H-W, Pan Y-CE, Lesslauer W, Brockhaus M: Purification and partial amino acid sequence analysis of two distinct tumor necrosis factor receptors from HL60 cells. J Biol Chem 265: 20131–20138, 1990
Thoma B, Grell M, Pfizenmaier K, Scheurich P: Identification of a 60-kD tumor necrosis factor (TNF) receptor as the major signal transducing component in TNF responses. J Exp Med 172:1019–1023, 1990
Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA Jr, Goeddel DV: The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci USA 88: 9292–9296, 1991
Tartaglia LA, Rothe M, Hu Y-F, Goeddel DV: Tumor necrosis factor's cytotoxic activity is signaled by the p55 TNF receptor. Cell 73: 213–216, 1993
Tartaglia LA, Ayres TM, Wong GHW, Goeddel DV: A novel domain within the 55 kd TNF receptor signals cell death. Cell 74: 845–853, 1993
Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld H-J, Broger C, Loetscher H, Lesslauer W: Crystal structure of the soluble human 55 kd TNF receptor-human TNFα complex: implications for TNF receptor activation. Cell 73: 431–445, 1993
Hsu H, Xiong J, Goeddel DV: The TNF receptor 1-associated protein TRADD signals cell death and NF-αB activation. Cell 81: 495–504, 1995
Rothe M, Wong SC, Henzel WJ, Goeddel DV: A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell 78: 681–692, 1994
Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D: A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem 270: 7795–7798, 1995
Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM: FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81: 505–512, 1995
Hsu H, Shu H-B, Pan M-G, Goeddel DV: TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84: 299–308, 1996
Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV: TNFdependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4: 387–396, 1996
Stanger BZ, Leder P, Lee TH, Kim E, Seed B: RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 81: 513–523, 1995
Boldin MP, Goncharov TM, Goltsev YV, Wallach D: Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1-and TNF receptor-induced cell death. Cell 85: 803–815, 1996
Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM: FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85: 817–827, 1996
Liu Z-G, Hsu H, Goeddel DV, Karin M: Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-αB activation prevents cell death. Cell 87: 565–576, 1996
Natoli G, Costanzo A, Ianni A, Templeton DJ, Woodgett JR, Balsano C, Levrero M: Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science 275: 200–203, 1997
Beg AA, Baltimore D: An essential role for NF-αB in preventing TNF-α-induced cell death. Science 274: 782–784, 1996
Wang C-Y, Mayo MW, Baldwin AS Jr: TNF-and cancer therapy-induced apoptosis: potentiation by inhibition of NF-αB. Science 274: 784–787, 1996
Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM: Suppression of TNF-α-induced apoptosis by NF-αB. Science 274: 787–789, 1996
Wallach D: Preparations of lymphotoxin induce resistance to their own cytotoxic effect. J Immunol 132: 2464–2469, 1984
Wong GHW, Goeddel DV: Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science 242: 941–944, 1988
Wong GHW, Elwell JH, Oberley LW, Goeddel DV: Manganase superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell 58: 923–931, 1989
Alexander RB, Nelson WG, Coffey DS: Synergistic enhancement by tumor necrosis factor of in vitro cytotoxicity from chemotherapeutic drugs targeted at DNA topoisomerase II. Cancer Res 47: 2403–2406, 1987
Krosnick JA, Mulé JJ, McIntosh JK, Rosenberg SA: Augmentation of antitumor efficacy by the combination of recombinant tumor necrosis factor and chemotherapeutic agents in vivo. Cancer Res 46: 3729–3733, 1989
Safrit JT, Bonavida B: Sensitivity of resistant human tumor cell lines to tumor necrosis factor and adriamycin used in combination: correlation between down-regulation of tumor necrosis factor-messenger RNA induction and overcoming resistance. Cancer Res 52: 6630–6637, 1992
Mizutani Y, Bonavida B: Overcoming cisdiamminedichloroplatinum (II) resistance of human ovarian tumor cells by combination treatment with cisdiamminedichloroplatinum (II) and tumor necrosis factoralpha. Cancer 72: 809–818, 1993
Uslu R, Bonavida B: Involvement of the mitochondrion respiratory chain in the synergy achieved by treatment of human ovarian carcinoma cell lines with both tumor necrosis factor-α and cis-diamminedichloroplatinum. Cancer 77: 725–732, 1996
Frost PJ, Belldegrun A, Bonavida B: Sensitization of immunoresistant prostate carcinoma cell lines to Fas/Fas ligand-mediated killing by cytotoxic lymphocytes: independence of de novo protein synthesis. Prostate 41: 20–30, 1999
Rosenberg B: Fundamental studies with cisplatin. Cancer 55: 2303–2316, 1985
Madajewicz S, Chowhan N, Tfayli A, Roque C, Meek A, Davis R, Wolf W, Cabahug C, Roche P, Manzione J, Iliya A, Shady M, Hentschel P, Atkins H, Braun A: Therapy for patients with high grade astrocytoma using intraarterial chemotherapy and radiation therapy. Cancer 88: 2350–2356, 2000
Sorenson CM, Eastman A: Mechanism of cisdiamminedichloroplatinum( ll)-induced cytotoxicity: role of G2 arrest and DNA double-strand breaks. Cancer Res 48: 4484–4488, 1988
Sorenson CM, Barry MA, Eastman A: Analysis of events associated with cell cycle arrest at G2 phase and cell death induced by cisplatin. J Natl Cancer Inst 82: 749–755, 1990
Poppenborg H, Knüpfer MM, Galla H-J, Ernst J, Wolff A: In vitro modulation of cisplatin resistance by cytokines. Cytokine 11: 689–695, 1999
Mashima T, Naito M, Kataoka S, Kawai H, Tsuruo T: Aspartate-based inhibitor of interleukin-1 α-converting enzyme prevents antitumor agent-induced apoptosis in human myeloid leukemia U937 cells. Biochem Biophys Res Commun 209: 907–915, 1995
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C: Anovel assay for apoptosis. Flowcytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184: 39–51, 1995
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987
Natoli G, Costanzo A, Guido F, Moretti F, Bernardo A, Burgio VL, Agresti C, Levrero M: Nuclear factor kBindependent cytoprotective pathways originating at tumor necrosis factor receptor-associated factor 2. J Biol Chem 273: 31262–31272, 1998
Rothe M, Sarma V, Dixit VM, Goeddel DV: TRAF2-mediated activation of NF-αB by TNF receptor 2 and CD40. Science 269: 1424–1427, 1995
Natoli G, Costanzo A, Guido F, Moretti F, Levrero M: Apoptotic, non-apoptotic, and anti-apoptotic pathways of tumor necrosis factor signalling. Biochem Pharmacol 56: 915–920, 1998
Tartaglia LA, Pennica D, Goeddel DV: Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J Biol Chem 268: 18542–18548, 1993
Higuchi M, Aggarwal BB: Differential roles of two types of the TNF receptor in TNF-induced cytotoxicity, DNA fragmentation, and differentiation. J Immunol 152: 4017–4025, 1994
Vandenabeele P, Declercq W, Vanhaesebroeck B, Grooten J, Fiers W: Both TNF receptors are required for TNF-mediated induction of apoptosis in PC60 cells. J Immunol 154: 2904–2913, 1995
Sherman SE, Wang DGAH-J, Lippard SJ: X-ray structure of the major adduct of the anticancer drug cisplatin with DNA: cis-[Pt(NH3){d(pGpG)}]. Science 230: 412–417, 1985
Calsou P, Salles B: Role of DNA repair in the mechanisms of cell resistance to alkylating agents and cisplatin. Cancer Chemother Pharmacol 32: 85–89, 1993
Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M: Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 352: 345–347, 1991
Van Meir E, Sawamura Y, Diserens A-C, Hamou M-F, de Tribolet N: Human glioblastoma cells release interleukin 6 in vivo and in vitro. Cancer Res 50: 6683–6688, 1990
Burrow ME, Weldon CB, Tang Y, Navar GL, Krajewski S, Reed JC, Hammond TG, Clejan S, Beckman BS: Differences in susceptibility to tumor necrosis factor alphainduced apoptosis among MCF-7 breast cancer cell variants. Cancer Res 58: 4940–4946, 1998
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Duan, L., Aoyagi, M., Tamaki, M. et al. Sensitization of Human Malignant Glioma Cell Lines to Tumor Necrosis Factor-induced Apoptosis by Cisplatin. J Neurooncol 52, 23–36 (2001). https://doi.org/10.1023/A:1010685110862
Issue Date:
DOI: https://doi.org/10.1023/A:1010685110862