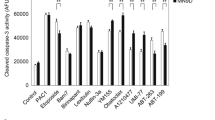
Abstract
Bcl-2 is a gene with clear anti-apoptotic properties in neurodegenerative conditions. One of the earliest hallmarks of degeneration in neuronal cell cultures is the loss of neurite morphology. Therefore the effect of Bcl-2 on neuronal morphology and microtubule stability was studied in nerve growth factor differentiated PC12 cells. Microtubule dynamics were modulated using the microtubule stabilizer taxol and the microtubule destabilizer, okadaic acid, a protein phosphatase inhibitor. It was shown that Bcl-2 protects against both taxol- and okadaic acid induced neurite retraction. Bcl-2 overexpression also significantly reduced the increased ratio of acetylated tubulin over total tubulin induced by taxol treatment. Interestingly, Bcl-2 attenuates the decrease of the same ratio after exposure to okadaic acid, suggesting that Bcl-2 is able to normalize the level of acetylated tubulin. In addition, cell death and nuclear fragmentation, induced by okadaic acid, were reduced in Bcl-2 overexpressing cells. This protection is either downstream or independent of tau phosphorylation as quantitative immunocytochemistry with AT8 showed that Bcl-2 did not modify the level of tau phosphorylation. The data suggest that the protective effect of Bcl-2 on the neuronal cytoskeleton is probably linked to changes in the post-translational modification of tubulin.
Similar content being viewed by others
References
Brown A, Li Y, Slaughter T, Black M. Composite microtubules of the axon: quantitative analysis of tyrosinated and acetylated tubulin along individual axonal microtubules. J Cell Sci 1993; 104: 339–352.
Audebert S, Desbruyeres E, Gruszczynski C, et al. Reversible polyglutamylation of alpha-and beta-tubulin and microtubule dynamics in mouse brain neurons. Mol Biol of the Cell 1993; 4: 615–626.
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder L. Abnormal phosphorylation of the microtubuleassociated protein tau in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 1986; 83: 4913–4917.
Nuydens R, de Jong M, Nuyens R, Cornelissen F, Geerts H. Neuronal kinase stimulation leads to aberrant tau phosphorylation and neurotoxicity. Neurobiol Aging 1995; 16: 465–477.
Xie H, Litersky JM, Hartigan JA, Jope RS, Johnson GVW. The interrelationship between selective tau phosphorylation and microtubule association. Brain Res 1998; 798: 173–183.
Merrick S, Demoise D, Lee V. Site-specific dephosphorylation of tau protein at ser 202/thr205 in response to microtubule depolymerization in cultured human neurons involves protein phosphatase 2A. J Biol Chem 1996; 271: 5589–5594.
Bonfoco E, Ceccatelli S, Manzo L, Nicotera P. Colchicine induces apoptosis in cerebellar granule cells. Exp Cell Res 1996; 218: 189–200.
Nuydens R, de Jong M, Van Den Kieboom G, Heers C, Dispersyn G, Cornelissen F, Nuyens R, Borgers M, Geerts H. Okadaic acid-induced apoptosis in neuronal cells: Evidence for an abortive mitotic attempt. J Neurochem 1998; 70: 1124–1133.
Mills J, Lee V, Pittman R. Activation of PP2A-like phosphatase and dephosphorylation of tau protein characterize onset of the execution phase of apoptosis. J Cell Sci 1998; 111: 625–636.
Sadot E, Barg J, Rasouly P, Lazarovici P, Ginzburg I. Shortand long-term mechanisms of tau-regulation in PC12 cells. J Cell Sci 1995; 108: 2857–2864.
Nuydens R, Dispersyn G, de Jong M, Van Den Kieboom G, Borgers M, Geerts H. Aberrant tau phosphorylation and neurite retraction during NGF deprivation in PC12 cells. Biochem Biophys Res Commun 1997; 240: 687–691.
Kroemer G. The proto-oncogene Bcl-2 and it's role in regulating apoptosis. Nat Med 1997; 3: 614–620.
Tanabe H, Eguchi Y, Kamada S, Martinou J, Tsujimoto Y. Susceptibility of cerebellar neurons derived from Bcl-2–deficient and transgenic mice to cell death. Eur J Neurosci 1997; 9: 848–856.
Steger C. An unbiased detector of curvilinear structures. IEEE Transactions on pattern analysis and machine intelligence 1998; 20(2): 113–125.
Schliwa M, Van Blerkom J. Structural interaction of cytoskeletal elements. J Cell Biol 1981; 90: 222–235.
Batistatou A and Greene L. Aurintricarboxylic acid rescues PC12 cells and sympathetic neurons from cell death caused by nerve growth factor deprivation: correlation with suppression of endonuclease activity. J Cell Biol 1991; 115: 461–471.
Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994; 124: 1–6.
Martinou J, Dubois-Dauphin M, Staple J, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 1994; 13: 1017–1030.
Sagot Y, Dubois-Dauphin M, Tan S, de Bilbao F, Aebischer P, Martinou J-C, Kato A. Bcl-2 overexpression prevents motorneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. J Neurosci 1995; 15: 7727–7733.
Panvichian R, Orth K, Day ML, Pilat MJ, Pienta KJ. Paclitaxel-associated multimininucleation is permitted by the inhibition of caspase activation: a potential early step in drug resistance. Cancer Res 1998; 58: 4667–4672.
Gliksman N, Parsons S, Salmon E. Okadaic acid induces interphase to mitotic-like microtubule dynamic instability by inactivating rescue. J Cell Biol 1992; 119(5): 1271–6.
Howell B, Odde D, Cassimeris L. Kinase and phosphatase inhibitors cause rapid alterations in microtubule dynamic instability in living cells. Cell Motil Cytoskeleton 1997; 38: 201–214.
Shea T, Fischer I. Phosphatase inhibition in neuroblastoma cells alters tau antigenicity and renders it incompetent to associate with exogenous microtubules. FEBS Lett 1996; 380: 63–67.
Sontag E, Nunbhakdi V, Bloom G, Mumby M. A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J Cell Biol 1995; 128: 1131–1144.
Merrick S, Trojanowski J, Lee V. Selective destruction of stable microtubules and axons by inhibitors of protein serine/ threonine phosphatases in cultured human neurons (NT2N cells). J Neurosci 1997; 17: 5726–5737.
Lesort M, Jope R, Johnson G. Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3B and fyn tyrosine kinase. J Neurochem 1999; 72: 576–584.
Alonso A, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA 1994; 91: 5562–5566.
Hempen B and Brion J. Reduction of acetylated alpha-tubulin immunoreactivity in neurofibrillary tangle bearing neurons in Alzheimer's disease. J Neuropath Exp Neurol 1996; 55(9): 964–972.
Tint I, Slaughter T, Fischer I, Black MI. Acute inactivation of Tau has no effect on dynamics of microtubules in growing axons of cultured sympathetic neurons. J Neurosci 1998; 18: 8660–8673.
Tanaka T, Zhong J, Iqbal K, Trenkner E, Grundke-Iqbal I. The regulation of phosphorylation of tau in SHSY neuroblastoma cells: the role of protein phosphatases. FEBS Lett 1998; 426: 248–254.
Poruchynski MS, Wang EE, Rudin CM, Blagosklonny MV, Fojo T. Bcl-XL is phosphorylated in malignant cells following microtubule disruption. Cancer Res 1998; 58: 3331–3338.
Geyp M, Ireland CM, Pitmann SM. Resistance to apoptotic cell death in a drug resistant T-cell leukaemia cell line. Leukemia 1996; 10: 447–455.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nuydens, R., Dispersyn, G., Van Den Kieboom, G. et al. Bcl-2 protects against apoptosis-related microtubule alterations in neuronal cells. Apoptosis 5, 43–51 (2000). https://doi.org/10.1023/A:1009685609275
Issue Date:
DOI: https://doi.org/10.1023/A:1009685609275