Abstract
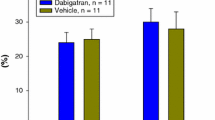
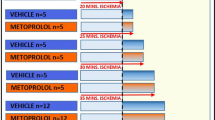
Poloxamer 188 is a surfactant with hemorheological, antithrombotic, and neutrophil-inhibitory properties. This agent has been demonstrated to reduce infarct size and to improve left ventricular function in animal models of myocardial infarction and reperfusion, and recently in a randomized trial of patients receiving thrombolytic therapy for acute myocardial infarction. In addition to reducing reperfusion injury, poloxamer 188 might be beneficial by increasing collateral blood flow. The purpose of this study was to determine the effect of poloxamer 188 on collateral blood flow, myocardial infarct size, and left ventricular function in a canine model of prolonged (3 hours) coronary occlusion and reperfusion. Closed-chest dogs (n = 21) underwent a 3-hour coronary occlusion and 3 hours of reperfusion. At 1 hour of occlusion, dogs received poloxamer 188, 75 mg/kg IV bolus, followed by 150 mg/kg/h IV for the final 2 hours of coronary occlusion and throughout reperfusion, or a saline placebo. Regional myocardial blood flow was measured using colored microspheres. Myocardial infarct size and area at risk were determined by postmortem histochemical staining. Compared with controls, poloxamer 188–treated dogs showed no significant increase in collateral blood flow during the final 2 hours of a 3-hour coronary artery occlusion. In addition, poloxamer 188 treatment had no beneficial effect on infarct size or left ventricular function in this model. Increased collateral blood flow is unlikely to be a beneficial mechanism of poloxamer 188 in myocardial infarction. These data also question the benefit of this agent to reduce reperfusion injury in the setting of more prolonged (3-hour) coronary occlusion.
Similar content being viewed by others
References
Carter C, Fisher TC, Hamai H, et al. Haemorheological effects of a nonionic copolymer surfactant (poloxamer 188). Clin Hemorheol 1992;12:109–120.
Hunter RL, Papadea C, Gallagher CJ, Finlayson DC, Check IJ. Increased whole blood viscosity during coronary artery bypass surgery: Studies to evaluate the effects of soluble fibrin and poloxamer 188. Thromb Haemostas 1990;63:6–12.
Grover FL, Kahn RS, Heron MW, Paton BC. A nonionic surfactant and blood viscosity: Experimental observations. Arch Surg 1973;106:307–310.
Gaehtgens P, Benner KU. Disaggregation of human red blood cells by various surface-active agents as related to changes of cell shape and hemolysis. Acta Haematol 1975;53:82–89.
Smith CM, Hebbel RP, Tukey DP, Clawson CC, White JG, Vercellotti GM. Pluronic F-68 reduces the endothelial adherance and improves the rheology of liganded sickle erythrocytes. Blood 1987;69:1631–1636.
Schaer GL, Hursey TL, Abrahams SL, et al. Reduction in reperfusion-induced myocardial necrosis in dogs by RheothRx injection (poloxamer 188, N.F.), a hemorheological agent that alters neutrophil function. Circulation 1994;90:2964–2975.
Schaer GL, Moy JN, Hursey TL, Rajwani KS, Thota V, Parrillo JE. Neutrophil function is altered by RheothRx injection (poloxamer 188), a hemorheologic agent shown to reduce reperfusion injury (abstr). J Am Coll Cardiol 1994;23:198.
Justicz AG, Farnsworth WV, Soberman MS, et al. Reduction of myocardial infarct size by poloxamer 188 and mannitol in a canine model. Am Heart J 1991;122:671–680.
Schaer GL, Spaccavento LJ, Browne KF, et al. Beneficial effects of RheothRx Injection in patients receiving thrombolytic therapy for aute myocardial infarction. Circulation 1996;94:298–307.
O'Keefe JH Jr., Grines CL, DeWood MA, et al. Poloxamer-188 as an adjunct to primary percutaneous transluminal coronary angioplasty for acute myocardial infarction. Am J Cardiol 1996;78:747–750.
Weaver WD. Randomized, placebo controlled trial of RheothRx (poloxamer 188) injection in patients with suspected acute myocardial infarction (AMI) (abstr). Circulation 1995;92:I24.
Collaborative Organization for RheothRx Evaluation (CORE). Effects of RheothRx on mortality, morbidity, left ventricular function, and infarct size in patients with acute myocardial infarction. Circulation 1997;96:192–201.
Hansen PR. Role of neutrophils in myocardial ischemia and reperfusion. Circulation 1995;91:1872–1885.
Entman ML, Michael L, Rossen RD, et al. Inflammation in the course of early myocardial ischemia. FASEB J 1991;5:2529–2537.
Kloner RA. Does reperfusion injury exist in humans? J Am Coll Cardiol 1993;21:537–545.
Carr ME Jr., Powers PL, Jones MR. Effects of poloxamer 188 on the assembly, structure, and dissolution of fibrin clots. Thromb Haemost 1991;66:565–568.
Hunter RL, Bennett B, Check IJ. The effect of poloxamer 188 on the rate of in vitro thrombolysis mediated by t-PA and streptokinase. Fibrinolysis 1990;4:117–123.
Reimer KA, Jennings RB, Cobb FR, et al. Animal models for protecting ischemic myocardium: Results of the NHLBI cooperative study. Circ Res 1985;56:651–665.
Habib GB, Heibig J, Forman SA, et al. Influence of coronary collateral vessels on myocardial infarct size in humans. Circulation 1991;83:739–746.
Lesnefsky EJ, Van Benthuysen KM, McMurtry IF, Shikes RH, Johnston RB Jr., Horwitz LD. Lidocaine reduces canine infarct size and decreases release of a lipid peroxidation product. J Cardiovasc Pharmacol 1989;13:895–901.
Sandler H, Dodge HT. The use of single plane angiocardiograms for the calculation of left ventricular volume in man. Am Heart J 1968;75:325–334.
Sheehan FH, Bolson EL, Dodge HT, Mathey DG, Schofer J, Hok-Wai W. Advantages and applications of the centerline method for characterizing regional ventricular function. Circulation 1986;74:293–305.
Hale SL, Alker KJ, Kloner RA. Evaluation of nonradioactive, colored microspheres for measurement of regional myocardial blood flow in dogs. Circulation 1988;78:428–434.
Cronstein BN, Levin RI, Belanoff J, Weissmann G, Hirschhorn R. Adenosine: An endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest 1986;78:760–770.
Romson JL, Bush LR, Jolly SR, Lucchesi BR. Cardioprotective effects of ibuprofen in experimental regional and global myocardial ischemia. J Cardiovasc Pharmacol 1982;4: 187–196.
Schaer GL, Karas SP, Santoian EC, Gold C, Visner MS, Virmani R. Reduction in reperfusion injury by blood-free reperfusion after experimental myocardial infarction. J Am Coll Cardiol 1990;15:1385–1393.
Vivaldi MT, Kloner RA, Schoen FJ. Triphenyltetrazolium staining of irreversible ischemic injury following coronary artery occlusion in rats. Am J Pathol 1985;121:522–530.
Robinson KA, Hunter RL, Stack JE, Hearn JA, Apkarian RP, Roubin GS. Inhibition of coronary arterial thrombosis in swine by infusion of poloxamer 188. J Invas Cardiol 1990;2:9–20.
Carr ME Jr., Zeker SL, Jones MR. Effects of RheothRx on clot formation in platelet rich plasma (abstr). Blood 1990;76(Suppl. 1):415a.
Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest 1974;54:1496–1508.
Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. N Engl J Med 1993;328:673–679.
Shen YT, Knight DR, Canfield DR, Vatner SF, Thomas JX Jr. Progressive change in collateral blood flow after coronary occlusion in conscious dogs. Am J Physiol 1989;256: H478–H485.
Schwartz H, Leiboff RH, Bren GB, et al. Temporal evolution of the human coronary collateral circulation after myocardial infarction. J Am Coll Cardiol 1984;4:1088–1093.
Rentrop KP, Feit F, Sherman W, Thornton JC. Serial angiographic assessment of coronary artery obstruction and collateral flow in acute myocardial infarction. Report from the second Mount Sinai-New York University reperfusion trial. Circulation 1989;80:1166–1175.
Gallagher KP, Buda AJ, Pace D, Gerren RA, Shlafer M. Failure of superoxide dismutase and catalase to alter size of infarction in conscious dogs after 3 hours of occlusion followed by reperfusion. Circulation 1986;73:1065–1076.
Babbitt DG, Virmani R, Vildibill HD Jr., Norton ED, Forman MB. Intracoronary adenosine administration during reperfusion following 3 hours of ischemia: Effects on infarct size, ventricular function, and regional myocardial blood flow. Am Heart J 1990;120:808–818.
Jolly SR, Kane WJ, Hook BG, Abrams GD, Kunkel SL, Lucchesi BR. Reduction of myocardial infarct size by neutrophil depletion: Effect of duration of occlusion. Am Heart J 1986;112:682–690.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kelly, R.F., Hursey, T.L., Patel, R.B. et al. Effect of Poloxamer 188 on Collateral Blood Flow, Myocardial Infarct Size, and Left Ventricular Function in a Canine Model of Prolonged (3-Hour) Coronary Occlusion and Reperfusion. J Thromb Thrombolysis 5, 239–247 (1998). https://doi.org/10.1023/A:1008848026759
Issue Date:
DOI: https://doi.org/10.1023/A:1008848026759