Abstract
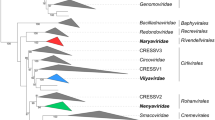
The complete nucleotide sequence of the triple gene block one (TGB 1) of cymbidium mosaic potexvirus (CymMV) was compared to those from other potex-, carla-, furo- and hordeiviruses. Seven conserved motifs in the TGB 1, including the ATP-GTP binding domain (P-Loop) consensus GXXGKTSTS, were found in all four virus genera. We propose that all TGBV can be classified into phylogenetic clusters based on their TGB 1 homolog genes. These clusters can be further delineated to form subgroups. The first cluster comprises the potexviruses which are further subdivided into three subgroups; BaMV, FMV, PlaMV and PapMV (subgroup Ia); CymMV, PAMV, NMV, SMYEaV and WClMV (subgroup Ib) and PVX (subgroup Ic). The second cluster comprises carlaviruses with a dual subgrouping; CVB, LSV, PVM, PMV and ASPV (subgroup IIa) and LVX (subgroup IIb). The third cluster carries the most diverse of TGBV comprising furoviruses PCV, PMTV and BSBV (subgroup IIIa) and hordeiviruses PSLV, BSMV and LRSV (subgroup IIIb). The phylogenetic relationships of triple gene block viruses (TGBV) based on the TGB 1 homolog gene indicates a convergent evolution.
Similar content being viewed by others
References
Maule A.J., Trends in Microbiol 2, 205-206, 1994.
Atabekov J.G. and Taliansky M.E., Adv in Virus Res 38, 201-247, 1990.
DeJong W., Chua A., and Ahlquist P., Virology 214, 464-474, 1995.
Fenczik C.A., Padgett H.S., Holt C.A., Caspar C.J., and Beachy R.N., MPPI 8, 666-673, 1995.
Forster R.L.S., Bevan M.W., Harbison S.A., and Gardner R.L., Nucleic Acids Res 16, 291-303, 1988.
Beck D.L., Guilford P.J., Voot D.M., Andersen M.T., and Forster R.L.S., Virology 183, 695-702, 1991.
Morozov S.Y., Fedorkin O.N., Juttner G., Schiemann J., Baulcombe D.C., and Atabekov J.G., J Gen Virol 78, 2077-2083, 1997.
Gilmer D., Bouzoubaa S., Henn A., Guilley H., Richards K., and Jonard G., Virology 189, 40-47, 1992.
Petty I.T.D. and Jackson A.O., Virology 179, 712-718, 1990.
Rouleau M., Smith R.J., Bancroft J.B., and Mackie G.A., Virology 204, 254-265, 1994.
Malyshenko S.I., Kondakova O.A., Taliansky M.E., and Atabekov J.G., J Gen Virol 70, 2751-2757, 1989.
Wong S.M., Mahtani P.H., Lee K.C., Yu H.H., Tan Y., Neo K.K., Chan Y., Wu M., and Chng CG, Arch Virol 142, 383-391, 1997.
Wong S.M., Chng C.G., Lee Y.H., Tan K., and Zettler F.W., Crop Prot 13, 235-239, 1994.
Frowd J.A. and Tremaine J.H., Phytopathology 67, 43-49, 1977.
Felsenstein J., PHYLIP (Phylogenetic Inference Package) Version 3.57c, Department of Genetics, University of Washington, Seattle, W.A., U.S.A., 1995.
Thomson J.D., Higgins D.G., and Gibson T.J., Nucleic Acids Res 22, 4673-4680, 1994.
Neo K.K., Wong S.M., and Wu M., Plant Mol Biol 18, 1027-1029, 1992.
Koonin E.V. and Dolja V.V., Cri Rev Biochem Mol Biol 28, 375-430, 1993.
Habili N. and Symons R.H., Nucleic Acids Res 17, 9543-9555, 1989.
Eagles R.M., Balmori-Meilan E., Beck D.L., Gardner R.C., and Forster R.L.S., Eur J Biochem 224, 667-684, 1994.
Ferabdez A., Lain S., and Garcia J.A., Nucleic Acids Res 17, 8413-8440, 1995.
Jin L. and Peterson D.L., Arch Biochem Biophy 323, 47-53, 1995.
Bleykasten C., Gilmer D., Guilley G.H., Richards K.E., and Jonard G., J Gen Virol 77, 889-897, 1996.
Solovyev A.G., Savenkov E.I., Agranovsky A.A., and Morozov S.Y., Virology 219, 9-18, 1996.
Torrance L. and Mayo M.A., Arch Virol 142, 435-439, 1997.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wong, SM., Lee, KC., Yu, HH. et al. Phylogenetic Analysis of Triple Gene Block Viruses Based on the TGB 1 Homolog Gene Indicates a Convergent Evolution. Virus Genes 16, 295–302 (1998). https://doi.org/10.1023/A:1008034807216
Issue Date:
DOI: https://doi.org/10.1023/A:1008034807216