Abstract
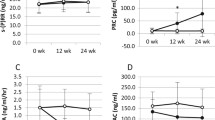
Increased sympathetic activity seems to play an important role in the pathogenesis and development of complications of atherosclerotic origin in patients with essential hypertension (EH). The aim of this study was to evaluate the effect of a new antihypertensive agent, moxonidine (M), on microalbuminuria (urine albumin excretion, UAE), plasma thrombomodulin (TM), and tissue plasminogen activator inhibitor (PAI-1) in patients with mild to moderate EH associated with increased UAE. Fifty-eight patients (32 M, 26 F) with EH and microalbuminuria, with a mean age of 56.6 ± 8.2 years and a body mass index (BMI) of 23.8 ± 3.1 kg/m2 who responded to M therapy (0.3–0.4 mg/daily) were studied before and after their blood pressure control. The 24-hour urine albumin excretion (RIA method), as well as TM and PAI-1 plasma levels (ELISA method), were determined before and 6 months after the initiation of treatment under moxonidine therapy. At the end of the 6-month period, all patients remained normotensive. The 24-hour urine albumin excretion had decreased to 24.5 ± 6.4 vs. 32.3 ± 7.2 ug/min before therapy (P < 0.001). The plasma TM levels had decreased to 44.0 ± 7 vs. 51.0 ± 9 ng/mL before therapy (P < 0.01), and PAI-1 levels had also decreased to 11.5 ± 4.5 vs. 15.8 ± 8 IU/mL before therapy (P < 0.05). The results of our study suggest that in hypertensive patients with microalbuminuria, moxonidine, an imidazoline I1-receptor agonist, a new centrally acting antihypertensive agent, significantly reduces urine albumin excretion as well as thrombomodulin and PAI-1 levels. These preliminary findings demonstrate a favorable effect on renal function and endothelial homeostatic mechanisms (maintenance of haemostatic balance).
Similar content being viewed by others
References
Bigazzi R, Bianchi S, Campese VM, Baldani G. Prevalence of microalbuminuria in a large population of patients with mild to moderate essential hypertension. Nephron 1992; 61:94-97.
Sammerson JH, Bell RA, Koney JC. Racial differences in prevalence of microalbuminuria in hypertension. Am J Kidney Dis 1995;26:577-579.
Parwing HH, Gyntelberg F. Transcapillary escape rate of albumin and plasma volume in essential hypertension. Circ Res 1973;33:643-653.
Yudkin JS, Forrest RD, Jackson CA. Microalbuminuria predictor of vascular disease in nondiabetic subjects. Lancet 1988;2:531-534.
Ruilope LM, Rodicio JL. Microalbuminuria in clinical practice. Kidney Curr Surv, World Literature 1995;4:211-216.
Kuuisto J, Mykkayen L, Pyorala K, Laakso M. Hyperinsulinemic microalbuminuria, a new risk indicator for coronary heart disease. Circulation 1995;91:831-837.
Damsgaard EM, Froland A, Jorgensen OD, Mogensen CE. Microalbuminuria as predictor of increased mortality in elderly people. Br Med J 1990;300:297-300.
Jaffe EA. Cell biology of endothelial cells. Hum Pathol 1987;18:234-239.
Borsum T. Biochemical properties of vascular endothelial cells. Virchows Arch (B), Cell Pathol 1991;60:279-286.
Giddings JC. Endothelial cell biology and haemostasis. Br J Biomed Sci 1993;50:43-47.
Pearson JD. Vessel wall interactions regulating thrombosis. Br Med Bull 1994;50:776-788.
Blann DA, Taberner AD. A reliable marker of endothelial cell dysfunction: Does it exist? Br J Haematol 1995;90: 244-248.
Parwing HH. Microalbuminuria in essential hypertension and diabetes mellitus. J Hypertens 1996;14(Suppl. 2): S89-S94.
Luscher TF, Noll G. Endothelial function as an end-point in interventional trials. J Hypertens 1996;14(Suppl. 2): S111-S121.
Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure. The fifth report of the Joint National Committee on Detection, Evaluation of High Blood Pressure (JNC-V). Arch Intern Med 1993;153:154-83.
Pedrinelli R, Di Bello VA, Catapano G, et al. Microalbuminuria is a marker of left ventricular hypertophy but not hyperinsulinemia in nondiabetic atherosclerotic patients. Atherosclerosis 1993;13:900-906.
Viberti GC. Mechanisms of diabetic renal and cardiovascular disease. Acta Diabetol 1990;27:267-276.
European Concerted Action on Thrombosis and Disabilities (ECAT). Euglobulin clot lysis time. In: Jespersen J, Bertina RM, Haverkate F. Kluwe, eds. ECAT Assay Procedures Handbook. Dordrecht, 1992:125-129.
Amiral J, Adam M, Mimilia F, Larrivaz I, Chambrette B, Boffa MC. A new assay for soluble forms of thrombomodulin in plasma. Thromb Haemost 1991;65:947.
United States Renal Data System: VSRDS 1991. Annual Data Report. Bethesda, MD. National Institute of Diabetes and Digestive and Kidney Diseases, 1992.
Valderrabano F, Jones EHP, Mallick NP. Report of management of renal failure in Europe. Nephrol Dial Transplant 1995;10(Suppl. 5):1-25.
Rodicio JL. Does antihypertensive therapy protect the kidney in essential hypertension? J Hypertens 1996;14(Suppl. 2):S69-S76.
Parving HH, Morgensen CE, Jensen HAE, Ervin PE. Increased urinary albumin excretion rate in benign essential hypertension. Lancet 1974;I:1190-1192.
Redon J, Liao Y, Lozano JV, Miralles A, Baldo E, Cooper RS. Factors related to the presence of microalbuminuria in essential hypertension. Am J Hypertens 1994;7:801-807.
Cerasola G, Cottone S, D'lgnoto G et al. Microalbuminuria as a predictor of cardiovascular damage in essential hypertension. J Hypertens 1989;7(Suppl. 6):S332-S333.
Bianchi S, Bigazzi R, Baldori G, Campese VM. Microalbuminuria in patients with essential hypertension: Effects of several antihypertensive drugs. AmJ Med 1992; 93:525-528.
Erley C, Haefete V, Heyne N, Baran N, Rister T. Microalbuminuria in essential hypertension: Reduction by different antihypertensive drugs. Hypertension 1993;21:810-815.
Rosen P, Ohly P, Gleichmann H. Experimental benefit of moxonidine on glucose metabolism and insulin secretion in the fructose-fed rat. J Hypertens 1997;15(Suppl. 1):S31-S38.
Ruilope LM, Alcazar JM, Hernandez E, et al. Long-term influences of antihypertensive therapy on microalbuminuria in essential hypertension. Kidney Int 1994;45(Suppl. 45):S171-S173.
Hamsten A. The haemostatic system and coronary heart disease. Thromb Res 1993;70:1-38.
Pearson JD. Vessel wall interactions regulating thrombosis. Br Med Bull 1994;50:776-788.
Landin K, Tengborn L, Smith U. Elevated fibrinogen and plasminogen activator inhibitor (PAI-1) in hypertension are related to metabolic risk factors for cardiovascular disease. J Intern Med 1990;227:273-278.
Jansson JH, Johanson B, Boman K, et al. Hypofibrinolysis in patients with hypertension and elevated cholesterol. J Intern Med 1991;229:309-316.
Van Wersch JW, Rompelberg-Lahaye J, Lustermans FA. Plasma concentrations of coagulation and fibrinolysis factors and platelet function in hypertension. Eur J Clin Chem Clin Biochem 1991;29:375-379.
Glueck CJ, Glueck HI, Hamer T, et al. Beta blockers, Lp(a), hypertension and reduced basal fibrinolytic activity. Am J Med Sci 1994;307:317-324.
Gader AM, da Costa J, Parker SM et al. The systemic release of plasminogen activator to intravenous adrenaline in man: Dose response studies. Thromb Res 1973;3:51-57.
Ishi H, Majerus PW. Thrombomodulin is present in human plasma and urine. J Clin Invest 1985;76:2178-2184.
Seigneur M, Dufourcq P, Conri C, et al. Levels of plasma thrombomodulin are increased in atheromatous arterial disease. Thromb Res 1993;71:423-431.
Makris T, Krespi P, Votteas V, et al. Thrombomodulin and TFPI levels in patients with arterial hypertension. Eur Heart J 1997;18(Suppl. 599):85.
Takahashi H, Tatewaki W, Wada K, et al. DDAVP does not induce the release of thrombomodulin from endothelial cells. Thromb Haemost 1991;65:451.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krespi, P.G., Makris, T.K., Hatzizacharias, A.N. et al. Moxonidine Effect on Microalbuminuria, Thrombomodulin, and Plasminogen Activator Inhibitor-1 Levels in Patients with Essential Hypertension. Cardiovasc Drugs Ther 12, 463–467 (1998). https://doi.org/10.1023/A:1007702132210
Issue Date:
DOI: https://doi.org/10.1023/A:1007702132210