Abstract
Purpose. The objectives of this work were to evaluate the importance of moderate passive permeability on apparent P-glycoprotein (P-gp) kinetics, and demonstrate that inspection of basolateral to apical and apical to basolateral (BL-AP/AP-BL) permeability ratios may result in a compound being overlooked as a P-gp substrate and inhibitor of another drug's transport via P-gp inhibition.
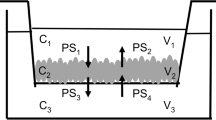
Methods. The permeability ratios of nicardipine, vinblastine, cimetidine, and ranitidine were determined across Caco-2 monolayers that express P-gp, in the presence and absence of the specific P-gp inhibitor, GF120918. In addition, the permeability ratio of vinblastine was studied after pretreatment of Caco-2 monolayers with nicardipine, ranitidine, or cimetidine. Similar studies were repeated with hMDR1-MDCK monolayers.
Results. The permeability ratios for cimetidine and vinblastine were >2. The permeability ratios for nicardipine and ranitidine were close to unity, and were not affected by the addition of GF120918. Based solely on ratios, only compounds with moderate transcellular permeability (vinblastine and cimetidine) would be identified as P-gp substrates. Although the permeability ratios appeared to be unity for nicardipine and ranitidine, both compounds affected the permeability of vinblastine, and were identified as substrates and inhibitors of P-gp. Studies performed in hMDR1-MDCK cells confirmed these experimental results. Data were explained in the context of a kinetic model, where passive permeability and P-gp efflux contribute to overall drug transport.
Conclusions. Moderate passive permeability was necessary for P-gp to reduce the AP-BL drug permeability. Inspection of the permeability ratio after directional transport studies did not effectively identify P-gp substrates that affected the P-gp kinetics of vinblastine. Because of the role of passive permeability, drug interaction studies with known P-gp substrates, rather than directional permeability studies, are needed to elucidate a more complete understanding of P-gp kinetics.
Similar content being viewed by others
REFERENCES
G. A. Altenberg, C. G. Vanoye, J. K. Horton, and L. Reuss. Unidirectional fluxes of rhodamine 123 in multidrug-resistant cells: evidence against direct drug extrusion from the plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 91:4654–4657 (1994).
M. Ramachandra, S. V. Ambudkar, D. Chen, C. A. Hrycyna, S. Dey, M. M. Gottesman, and I. Pastan. Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry 37:5010–5019 (1998).
A. B. Shapiro and V. Ling. Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur. J. Biochem. 250:130–137 (1997).
W. D. Stein, C. Cardarelli, I. Pastan, and M. M. Gottesman. Kinetic evidence suggesting that the multidrug transporter differentially handles influx and efflux of its substrates. Mol. Pharmacol. 45:763–772 (1994).
G. D. Eytan, R. Regev, G. Oren, C. D. Hurwitz, and Y. G. Assaraf. Efficiency of P-glycoprotein-mediated exclusion of rhodamine dyes from multidrug-resistant cells is determined by their passive transmembrane movement rate. Eur. J. Biochem. 248: 104–112 (1997).
G. D. Eytan, R. Regev, G. Oren, and Y. G. Assaraf. The role of passive transbilayer drug movement in multidrug resistance and its modulation. J. Biol. Chem. 271:12897–12902 (1996).
R. Evers, N. H. Cnubben, J. Wijnholds, L. van Deemter, P. J. van Bladeren, and P. Borst. Transport of glutathione prostaglandin A conjugates by the multidrug resistance protein 1. FEBS Lett. 419: 112–116 (1997).
K. A. Lentz, J. Hayashi, L. J. Lucisano, and J. E. Polli. Development of a more rapid, reduced serum culture system for Caco-2 monolayers and application to the Biopharmaceutics Classification System. Int. J. Pharm. 200:41–51 (2000).
J. W. Polli, J. E. Humphreys, S. A. Wring, T. C. Burnette, K. D. Read, A. Hersey, D. Butina, L. Bertolotti, P. Pugnaghi, and C. S. Serabjit-Singh. Comparison of MDCK and bovine brain endothelial cells (BBECs) as a blood-brain barrier screen in early drug development. In M. Balls, A. VanZeller, and M. Halder (eds.), Progress in the Reduction, Refinement and Replacement of Animal Experimentation, Elsevier Science, New York, 2000, pp. 271-289.
N. F. Ho, P. S. Burton, R. A. Conradi, and C. L. Barsuhn. A biophysical model of passive and polarized active transport processes in Caco-2 cells: approaches to uncoupling apical and basolateral membrane events in the intact cell. J. Pharm. Sci. 84: 21–27 (1995).
J. D. Irvine, L. Takahashi, K. Lockhart, J. Cheong, J. W. Tolan, H. E. Selick, and J. R. Grove. MDCK (Madin-Darby canine kidney) cells: A tool for membrane permeability screening. J. Pharm. Sci. 88:28–33 (1999).
S. Doppenschmitt, H. Spahn-Langguth, C. G. Regardh, and P. Langguth. Role of P-glycoprotein-mediated secretion in absorptive drug permeability: An approach using passive membrane permeability and affinity to P-glycoprotein. J. Pharm. Sci. 88: 1067–1072 (1999).
K. Ito, H. Kusuhara, and Y. Sugiyama. Effects of intestinal CYP3A4 and P-glycoprotein on oral drug absorption–theoretical approach. Pharm. Res. 16:225–231 (1999).
R. L. Shepard, M. A. Winter, S. C. Hsaio, H. L. Pearce, W. T. Beck, and A. H. Dantzig. Effect of modulators on the ATPase activity and vanadate nucleotide trapping of human Pglycoprotein. Biochem. Pharmacol. 56:719–727 (1998).
G. A. Sawada, L. R. Williams, B. S. Lutzke, and T. J. Raub. Novel, highly lipophilic antioxidants readily diffuse across the blood-brain barrier and access intracellular sites. J. Pharm. Exp. Ther. 288:1327–1333 (1999).
S. Ito, C. Woodland, B. Sarkadi, G. Hockmann, S. Walker, and G. Koren. Modeling of P-glycoprotein-involved epithelial drug transport in MDCK cells. Am. J. Physiol. 277:F84–F96 (1999).
J. Hunter, B. H. Hirst, and N. L. Simmons. Drug absorption limited by P-glycoprotein-mediated secretory drug transport in human intestinal epithelial Caco-2 cell layers. Pharm. Res. 10: 743–749 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lentz, K.A., Polli, J.W., Wring, S.A. et al. Influence of Passive Permeability on Apparent P-glycoprotein Kinetics. Pharm Res 17, 1456–1460 (2000). https://doi.org/10.1023/A:1007692622216
Issue Date:
DOI: https://doi.org/10.1023/A:1007692622216