Abstract
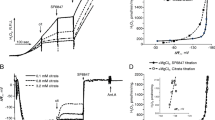
Zajdela hepatoma mitochondria were able to accumulate two to five times more Ca2+ than rat liver mitochondria before the permeability transition was induced. Pulses of Ca2+ were given in series to determine the Ca2+ threshold by recording changes in [Ca2+] and membrane potential, the permeability transition causing the release of accumulated Ca2+ and collapse of the membrane potential. Hepatoma mitochondria had lower Ca2+ efflux rates, higher net Ca2+ uptake rates and lower phosphorylation rates than liver mitochondria. Since the differences in regard to induction of the permeability transition might be due to higher expression of the Bcl-2 protein in hepatoma cells than in hepatocytes, the transcription of Bcl-2 and the proteins reacting with a Bcl-2 polyclonal antiserum were estimated by Northern and Western blotting, respectively. Hepatoma cells had two Bcl-2 specific mRNA bands of 7 and 2.4 kb, and substantial amounts of the Bcl-2 protein, whereas in liver cells and mitochondria these were not detected. Both cell lines had a reactive band at 19-20 kDa, and hepatocytes a small band at 31-32 kDa. Bcl-2 antibodies stimulated the permeability transition potently in hepatoma mitochondria.
Similar content being viewed by others
References
Williams TG: Programmed cell death: Apoptosis and oncogenesis. Cell 65: 1097–1098, 1991
Reed JC, Miyashita T, Takayama S, Wang H-G, Sato T, Rajewsi S, Aime-Sempe C, Bodrug S, Kitada S, Hanada M: BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem 60: 23–32, 1996
Fisher DE: Apoptosis in cancer therapy: crossing the threshold. Cell 78: 539–542, 1994
Kroemer G: The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nature Med 6: 614–620, 1997
Hockenbery DM, Zutter M, Hickey W, Nahm M, Korsmeyer SJ: BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death, Proc Natl Acad Sci USA 88: 6961–6965, 1991
Monaghan P, Robertson D, Amos TAS, Dyer MJS, Mason DY, Greaves MF: Ultrastructural localization of BCL-2 protein. J Histochem Cytochem 40: 1819–1825, 1992
Riparbelli MG, Callaini G, Tripodi SA, Cintorino M, Tosi P, Dallai R: Localization of the Bcl-2 protein to the outer mitochondrial membrane by electron microscopy. Exp Cell Res 221: 363–369, 1995
Boise LH, Gottschalk AR, Quintans J, Thompson CB: Bcl-2 and Bcl-2-related proteins in apoptosis regulation. Curr Top Microbiol Immunol 200: 107–121, 1995
Marchetti P, Hirsch T, Zamzami N, Castedo M, Decaudin D, Susin SA, Masse B, Kroemer G: Mitochondrial permeability transition triggers lymphocyte apoptosis. J Immunol 157: 4830–4836, 1996
Petit PX, Susin S-A, Zamzami N, Mignotte B, Kroemer G: Mitochondria and programmed cell death: Back to future. FEBS Lett 396. 7–13, 1996
Murphy AN, Brodesen DE, Cortopassi G, Wang E, Fiskum G: Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc Natl Acad Sci USA 93: 9893–9898, 1996
Leizt M, Nicotera P: The shape of cell death. Biochem Biophys Res Commun 236: 1–9, 1997
Gunter TE, Pfeiffer DR: Mechanisms by which mitochondria transport calcium. Am J Physiol 258: C755–786, 1990
Trump BF, Berezesky IK: Calcium-mediated cell injury and cell death. FASEB J 9. 219–228, 1995
Richter C, Schweizer M, Cossarizza A, Franceschi C: Control of apoptosis by the cellular ATP level. FEBS Lett 378: 107–110, 1996
Randeli W, Moreadith W, Fiskum G: Isolation of mitochondria from ascites tumor cells permeabilized with digitonin. Anal Biochem 137: 360–367, 1984
Johnson D, Lardy HA: Isolation of liver or kidney mitochondria. Meth Enzymol 10: 94–96, 1967
Saris N-EL, Allshire A: Calcium transport in mitochondria. Meth Enzymol 174: 68–84, 1989
Kamo N, Muratsugu M, Hongoh R, Kobatake Y: Membrane potential of mitochondria measured with an electrode sensitive to tetraphenylphosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol 49: 105–121, 1979
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage. Nature 4: 680–685, 1970
Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of protein from polyacrylamid gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354, 1979
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987
Sambrook J, Fretsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Second Edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1989
Zhao M, Zhang NX, Economou M, Blaha I, Laissue JA, Zimmermann A: Immunohistochemical detection of Bcl-2 protein is expressed in hepatocellular carcinomas but not in liver cell dysplasia. Histopathology 25: 237–245, 1994
Bygrave FL: Calcium transport in mitochondria isolated from normal and injured tissue. In: LJ Anghileri, AM Tuffet-Anghileri (eds). The Role of Calcium in Biological Systems. CRC Press Inc., Boca-Raton, Florida, 1982, 1, pp 121–145
Zinchenko VP, Teplova VV, Evtodienko Yu V: Membrane-bound calcium in the mitochondria of Ehrlich ascites cells. Bull Exp Biol Med (in Russian) 11: 563–566, 1985
Saris N-EL, Teplova VV, Azarashvili TS, Evtodienko Yu V, Virtanen I: The high calcium ion uptake capacity of Ehrlich ascites tumour cell mitochondria is due to inhibition of the permeability transition and phospholipase A2 activity by magnesium. Magnesium Res 11: 155–160, 1998
Evtodienko Yu V, Teplova V, Khawaja J, Saris N-EL: The Ca2+-induced permeability transition pore is involved in Ca2+-induced mitochondrial oscillations. A study on permeabilized Ehrlich ascites tumour cells. Cell Calcium 15: 1–10, 1994
Szabó I, Bernardi P, Zoratti M: Modulation of the mitochondrial megachannel by divalent cations and protons. J Biol Chem 267: 2940–2946, 1992
Hockenbery DM, Oltvai ZN, Yin X-M, Milliman CL, Korsmeyer SJ: Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75: 241–251, 1993
Benedict M, Tewari M, Shayman JA, Dixit VM: Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogen 10: 2297–2305, 1995
Richter C: Prooxidants and mitochondrial calcium: their relationship to apoptosis and oncogenesis. FEBS Lett 325: 104–107, 1993
Luciaková K, Kužela S: Increased content of natural ATPase inhibitor in tumor mitochondria. FEBS Lett 177: 85–88, 1984
Bogutska K, Teplova V, Wojtczak L, Evtodienko Yu: Inhibition by calcium of the hydrolysis and synthesis of ATP in Ehrlich ascites tumour mitochondria: relation to the Crabtree effect. Biochim Biophys Acta 1228: 261–266, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Evtodienko, Y.V., Teplova, V.V., Azarashvily, T.S. et al. The Ca2+ threshold for the mitochondrial permeability transition and the content of proteins related to Bcl-2 in rat liver and Zajdela hepatoma mitochondria. Mol Cell Biochem 194, 251–256 (1999). https://doi.org/10.1023/A:1006913526643
Issue Date:
DOI: https://doi.org/10.1023/A:1006913526643