Abstract
Purpose.It has been shown in vitro that both cisplatin and epirubicin increase the antitumor activity of paclitaxel. Weekly administration could give a substantial improvement in the therapeutic index of cisplatin and paclitaxel. This study was aimed at defining the antitumor activity of a weekly cisplatin–epirubicin–paclitaxel (PET) administration in locally advanced or metastatic breast cancer patients.
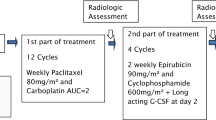
Patients and methods.Sixty-eight breast cancer patients with advanced disease, who had not received prior chemotherapy (except adjuvant), received weekly cisplatin 30 mg/sqm, paclitaxel 120 mg/sqm and epirubicin 50 mg/sqm plus G-CSF (day 3–5), for a maximum of 12 cycles. Thirty-five patients had stage IIIB and 33 stage IV disease (14 with visceral metastases).
Results.All patients were evaluable for response on an intent to treat basis. Overall, 21 complete and 38 partial responses have been recorded for an 87% ORR (95% CI = 76–94%). Fourteen CRs and 19 PRs have been registered in the 35 patients with locally advanced disease for a 94% ORR (95% CI = 81–99%) while 7 CRs and 19 PRs were observed in the 33 patients with metastatic disease for a 79% ORR (95% CI–61–91%). Surgery was performed in 33/35 women with locally advanced disease. Four of these patients (11%) showed no invasive cancer on pathologic examination, and in an additional 8 patients tumor < 1 cm was found in the breast. Only 4/33 patients who underwent surgery relapsed. The projected one-year RFS was greater than 80%. At an 11-month median follow-up (range, 3–19), 11 patients had progressed and 5 had died among the 33 patients with metastatic disease, the median progression-free survival in this group being 14 months. Severe hematologic toxicity was uncommon, grade 3–4 neutropenia and thrombocytopenia occurring in 32% and 4% of patients, respectively. Only 2 episodes of neutropenic sepsis were registered. Packed red blood cell transfusions were required in 7 patients. Vomiting, diarrhoea, mucositis and skin toxicity were severe in 6%, 9%, 10%, and 9% of patients, respectively. Peripheral neuropathy was observed in 47% of patients.
Conclusions.The weekly PET administration is a well tolerated and very effective approach in advanced breast cancer patients. It can produce a 40% clinical complete response rate, with a more than 10% pCR rate in patients with T4 disease, and an about 80% ORR in those with distant metastases. A phase III trial comparing PET with a standard every 3 weeks epirubicin—taxol administration is underway.
Similar content being viewed by others
References
Early Breast Cancer Trialists Collaborative Group: Polychemotherapy for early breast cancer: an overview of the randomized trials. Lancet 352: 930–942, 1998
Fossati R, Confalonieri C, Torri V, Ghislandi B, Penna A, Pistotti V, Tinazzi A, Liberati A: Cytotoxic and hormonal treatment for metastatic breast cancer. A systematic review of published randomized trials involving 31,510 women. J Clin Oncol 16: 3439–3460, 1998
Greenberg PA, Hortobagyi GN, Smith TL, Ziegler LD, Frye DK, Buzdar AU: Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol 14: 2197–2205, 1996
Holmes FA, Walters RS, Theriault RL, Forman AD, Newton LK, Raber MN, Budzar AU, Frye DK, Hortobagyi GN: Phase II trial of Taxol: An active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst 83: 1797–1805, 1991
Seidman AD, Reichman BS, Crown JP, Yao TJ, Currie V, Hakes TB, Hudis CA, Gilewski TA, Baselga J, Forsythe P, Lepore J, Marks L, Fain K, Souhrada M, Onetto N, Arbuck S, Norton L: Paclitaxel as second and subsequent therapy for metastatic breast cancer: Activity independent of prior anthracycline response. J Clin Oncol 13: 1152–1159, 1995
Chevallier B, Fumoleau P, Kerbrat P, Azli N, BayssasM, Lentz MA, van Glabbeke M, Dieras V, Roche H, Krakowski I: Docetaxel is a major cytotoxic drug for the treatment of advanced breast cancer: a phase II trial of the Clinical Screening Cooperative Group of the European Organization for Research and Treatment of Cancer. J Clin Oncol 13: 314–324, 1995
Seidman AD, Hudis CA, Albanel J, Tong W, Tepler I, Currie V, Moynahan M, Theodoulou M, Gollub M, Baselga J, Norton L: Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol 16: 3353–3361, 1998
Sikov W, Akerley W, Strenger R, Cummings F: Weekly highdose paclitaxel demonstrates significant activity in advanced breast cancer (abstract). Proc Am Soc Clin Oncol 17: 112a, 1998
Gianni L, Munzone E, Capri G, Fulfaro F, Tarenzi E, Villani F, Spreafico C, Laifranchi A, Caraceni, A, Martini C, Stefanelli M, Valagussa P, Bonadonna G: Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: High antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol 13: 2688–2699, 1995
Frassineti GL, Zoli W, Tienghi A, Ravaioli A, Milandri C, Gentile A, Onetto N, Arnadori D: Phase II study of sequential combination of paclitaxel and doxorubicin in the treatment of advanced breast cancer (abstract). Proc Am Soc Clin Oncol 15: 109, 1996
Schwartsmann G, Menke CH, Caleffi M, Xavier N, Ferreira Filho AF, Schunemann H, Koya R, Stroda PR, Pohlmann P, Vengas LF, Kalakun L: Phase II trial of taxol, doxorubicin plus G-CSF in patients with metastatic breast cancer (abstract). Proc Am Soc Clin Oncol 15: 126, 1996
Conte PF, Baldini E, Gennari A, Salvadori B, Gennari A, Da Prato M, Tibaldi C, Salzano E, Gentile A: Dose-finding study and pharrnacokinetics of epirubicin and paclitaxel over 3 hours, a regimen with high activity and low cardiotoxicity in advanced breast cancer. J Clin Oncol 15: 2510–2517, 1997
Sledge CW, Neuberg D, Ingle J, Martino S, Wood W: Phase III trial of doxorubicin (A) vs. paclitaxel (T) vs. doxorubicin– paclitaxel (A+T), as first-line therapy for metastatic breast cancer (MBC): an intergroup trial (abstract). Proc Am Soc Clin Oncol 16: la, 1997
Sledge GW, Loehrer PJ, Roth BJ, Einhom LH: Cisplatin as first-line therapy for metastatic breast cancer. J Clin Oncol 6: 1811–1814, 1988
Smith IE, Talbot DC: Cisplatin and its analogues in the treatment of advanced breast cancer. A review: Br J Cancer 65: 787–793, 1992
Dogliotti L, Danese S, Berruti A, Zola P, Bottini A, Buniva T, Richiardi G, Moro G, Farrris A, Porcile G, Zaffaroni N: Epirubicin, cisplatin and lonidamine in advanced breast cancer. A phase II study (abstract). Proc Am Soc Clin Oncol 15: 140, 1996
Roy SN, Horwitz SB: A phosphoglycoprotein associated with with taxol-resistance in J774.2 cells. Cancer Res 45: 3856–3863, 1985
Parker RJ, Eastman A, Bostick-Bruton F, Reed E: Acquired cisplatin resistance in human ovarian cancer cells is associated with enhanced repair of cisplatin–DNA lesions and reduced drug accumulation. J Clin Invest 87: 772–777, 1991
Frasci G, Comella P, D'Aiuto G, Budillon A, Barbarulo D, Thomas R, Capasso L, Casaretti R, Daponte A, Caponigro F, Gravina A, Carteni' G, Gentile A, Comella G. Weekly paclitaxel-cisplatin administration with G-CSF support in advanced breast cancer. A phase II study. Breast Cancer Res. Treat 49: 13–26, 1998
Gelmon KA, O'Reilly SE, Tolcher AW, Zola P, Bottini A, Buniva T, Richiardi G, Moro G, Farms A, Porcile G, Zaffaroni N: Phase I/II study of biweekly paclitaxel and cisplatin in the treatment of metastatic breast cancer. J Clin Oncol 14: 1185–1191, 1996
McCaskill-Stevens W, Ansari R, Fisher W, Pennington K, Dobbs C, Gonin R, Schaefer S, Loesch D, Sledge G: Phase II study of biweekly cisplatin and paclitaxel in the treatment of metastatic breast cancer (abstract). Proc Am Soc Clin Oncol 15: 120, 1996
Sparano JA, Neuberg D, Glick JH, Robert NJ, Goldstem U, Sledge GW, Wood W: A phase II trial of biweekly paclitaxel and cisplatin in patients with advanced breast carcinoma: an Eastern Cooperative Oncology Group trial. (abstract) Proc Am Soc Clin Oncol 15: 114, 1996
Frasci G, D'Aiuto G, Comella P, Apicella A, Thomas R, Capasso L, Di Bonito M, Carteni G, Biglietto M, De Lucia L, Maiorino L, Piccolo S, Bianchi U, D' Aniello R, Lapenta L, Comella G: Cisplatin–epirubicin–paclitaxel weekly administration in advanced breast cancer: A phase I study of the Southern Italy Cooperative Oncology Group. Breast Cancer Res Treat 56: 239–252, 1999
Miller AB, HQogstratep B, Staquet M: Reporting results of cancer treatment. Cancer 47: 207–214, 1981
Kaplan ES, and Meier P: Non parametric estimation for incomplete observations. J Am Stat Assoc 53: 557–580, 1958
Simon R, Wittes RE, Ellenberg SS: Randomised phase II clinical trials, Cancer Treat Rep 69: 1375–1381, 1985
Sola C, Lluch A, Garcia-Conde I, Ojeda B, Hornedo J, Benavides M, Solano C, Garcia T, Salazar R, Alonso S, Cortes-Funes H, Lopez JJ: Phase II study of weekly paclitaxel in recurrent breast cancer after high-dose chemotherapy (abstract). Proc Am Soc Clin Oncol 17: 174a, 1998
Alvarez A, Mickiewicz E, Brosio C, Giglio R, Cinat G, Suarez A: Weekly taxol in patients who had relapsed or remained stable with taxol in a 21 day schedule (abstract). Proc Am Soc Clin Oncol 17: 188a, 1998
Perez EA, Irwin DR, Patel R. et al.: A large phase II trial of paclitaxel administered as a weekly 1-hour infusion in patients with metastatic breast cancer. Proc Am Soc Clin Oncol 18: 126a, 1999
Kohler U, Olbricht SS, Fuechsel G, Keffner E, Richter B, Ridwelski K: Weekly paclitaxel with epirubicin as second-line therapy of metastatic breast cancer. Results of a clinical phase II study. Semin Oncol 24 (17) 40–43, 1997.
van der Burgh MEL, de Wit R, Stoter G, Verweij J: Phase I study of weekly cisplatin and weekly or 4-weekly taxol: a highly active regimen in advanced epithelial ovarian cancer (abstract) Proc Am Soc Clin Oncol 17: 355a, 1998
Bianco AR, DeLaurentis M, Carlomagno C. et al.: 20 year update of the Naples GUN trial of adjuvant breast cancer therapy: evidence of interaction between c-erbB2 expression and tamoxifen efficacy. Proc Am Soc Clin Oncol 17: 97a, 1998
Untch M, Konecny G, Lebeau A., et al.: Dose-intensification of anthracycline in the adjuvant treatment of high-risk breast cancer and c-erbB2 overexpression. Proc Am Soc Clin Oncol 17: 102a, 1998
Colomer R, Montere S, Lluch A, et al.: Circulating HER2/Neu predicts resistance to taxol/adriamycin in metastatic breast carcinoma: preliminary results of a multicentric prospective study. Proc Am Soc Clin Oncol 16: 140a, 1997
Norton L, Slamon D, Leyland-Jones B, Wolter J, Fleming T, Eirmann W, Baselga J, Mendelsohn J, Bajamonde A, Ash M, Shak S: Overall survival advantage to simultaneous chemotherapy plus humanized anti-HER 2 monoclonal antibody herceptin in HER2-overexpressing metastatic breast cancer. Proc Am Soc Clin Oncol 18: 127a, 1999
Fornier M, Seidman AD, Esteva FJ, Theodoulou M, Moynahan M, Currie V, Moasser M, Sklarin N, Gilewski T, Surbone A, Denton C, Bacoffi D, Willey J, Bach A, Reuter V, Hortobagyi G, Norton L, Hudis C: Weekly herceptin + 1-hr taxol: phase II study in HER2 overexpressing and nonoverexpressing metastatic breast cancer. Proc Am Soc Clin Oncol 18: 126a, 1999
Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz AB, Fisher ER, Wickerman DL, Wolmark N, DeCillis A, Hoehn JL, Lees AW, Dimitrov NY: Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15: 2483–2494, 1997
Moliterni A, Tarenzi E, Capri G, Terenziani M, Bertuzzi A, Grasselli G, Agresti R, Piotti P, Greco M, Salvadori B, Pilotti S, Lombardi F, Valagussa P, Bonadonna G, Gianni L: Pilot study of primary chemotherapy with doxorubicin plus paclitaxel in women with locally advanced or operable breast cancer. Semin Oncol 24(17) 10–14, 1997
Henderson IC, Berry G, Demetri C, Cirrincione C, Goldstein L, Martino S, Ingle JN, Cooper MR, Canellos G, Burderi F, Fleming G, Holland M, Graziano S, Carpenter J, Muss H, Norton L: Improved disease-free and overall survival from the addition of sequential paclitaxel but not from the escalation of doxorubicin dose level in the adjuvant chemotherapy of patients with node-positive primary breast cancer. Proc Am Soc Clin Oncol 17: l0la, 1998
Hudis C, Seidman A, Baselga J, Raptis J, Lebwohl D, Gilewski T, Moynahan M, Skarin N, Fennelly D, Crown PA, Surbone, A, Uhlenhopp M, Riedel F, Yao U, and Norton L: Sequential dose-dense doxorubicin, paclitaxel, and cyclophosphamide for resectable high-risk breast cancer: feasibility and efficacy. J Clin Oncol 17: 93–100, 1999
Rights and permissions
About this article
Cite this article
Frasci, G., D'Aiuto, G., Comella, P. et al. Cisplatin–epirubicin–paclitaxel weekly administration with G-CSF support in advanced breast cancer. A Southern Italy Cooperative Oncology group (SICOG) phase II study. Breast Cancer Res Treat 62, 87–97 (2000). https://doi.org/10.1023/A:1006429205363
Issue Date:
DOI: https://doi.org/10.1023/A:1006429205363