Abstract
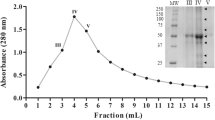
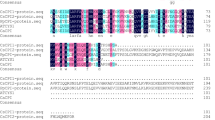
Cystatin CsC, a cysteine proteinase inhibitor from chestnut (Castanea sativa) seeds, has been purified and characterized. Its full-length cDNA clone was isolated from an immature chestnut cotyledon library. The inhibitor was expressed in Escherichia coli and purified from bacterial extracts. Identity of both seed and recombinant cystatin was confirmed by matrix-assisted laser desorption/ionization mass spectrometry analysis, two-dimensional electrophoresis and N-terminal sequencing. CsC has a molecular mass of 11 275 Da and pI of 6.9. Its amino acid sequence includes all three motifs that are thought to be essential for inhibitory activity, and shows significant identity to other phytocystatins, especially that of cowpea (70%). Recombinant CsC inhibited papain (Ki 29 nM), ficin (Ki 65 nM), chymopapain (Ki 366 nM), and cathepsin B (Ki 473 nM). By contrast with most cystatins, it was also effective towards trypsin (Ki 3489 nM). CsC is active against digestive proteinases from the insect Tribolium castaneum and the mite Dermatophagoides farinae, two important agricultural pests. Its effects on the cysteine proteinase activity of two closely related mite species revealed the high specificity of the chestnut cystatin.
Similar content being viewed by others
References
Abe K, Emori Y, Kondo H, Suzuki K, Arai S: Molecular cloning of a cysteine proteinase inhibitor of rice (oryzacystatin). J Biol Chem 262: 16793–16797 (1987).
Abe M, Abe K, Kuroda M, Arai S: Corn kernel cysteine proteinase inhibitor as a novel cystatin superfamily member of plant origin. Molecular cloning and expression studies. Eur J Biochem 209: 933–937 (1992).
Abe M, Arai S: Some properties of a cysteine proteinase inhibitor from corn endosperm. Agric Biol Chem 55: 2417–2418 (1991).
Barrett AJ: The cystatins: a new class of peptidase inhibitors. Trends Biochem Sci 12: 193–196 (1987).
Benchekroun A, Michaud D, Nguyen-Quoc B, Overney S, Desjardins Y, Yelle S: Synthesis of active oryzacystatin I in transgenic potato plants. Plant Cell Rep 14: 585–588 (1995).
Botella MA, Xu Y, Prabha TN, Zhao Y, Narasimhan ML, Wilson KA, Nielsen SS, Bressan RA, Hasegawa PM: Differential expression of soybean cysteine proteinase inhibitor genes during development and in response to wounding and methyl jasmonate. Plant Physiol 112: 1201–1210 (1996).
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 (1976).
Chang S, Puryear J, Cairney J: A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11: 113–116 (1993).
Dixon M, Webb EC, Throne CJR, Tipton KF: Enzyme inhibitors. In: Dixon M, Webb EC (eds) Enzymes, pp. 332–381. Academic Press, New York (1979).
Fernandes KVS, Campos FAP, Do Val RR, Xavier-Filho J: The expression of papain inhibitors during development of cowpea seeds. Plant Sci 74: 179–184 (1991).
Fernandes KVS, Sabelli PA, Barratt DHP, Richardon M, Xavier-Filho J, Shewry PR: The resistance of cowpea seeds to bruchid beetles is not related to levels of cysteine proteinase inhibitors. Plant Mol Biol 23: 215–219 (1993).
García-Olmedo F, Salcedo G, Sanchez-Monge R, Gomez L, Royo J, Carbonero P: Plant proteinaceous inhibitors of proteinases and _-amylases. Oxford Surv Plant Mol Cell Biol 4: 275–334 (1987).
Gruden K, Štrukelj B, Ravnikar M, Poljšak-Prijatelj M, Mavric I, Brzin J, Pungercar J, Kregar I: Potato cysteine proteinase inhibitor gene family: molecular cloning, characterisation and immunocytochemical localisation studies. Plant Mol Biol 34: 317–323 (1997).
Gutierrez C, García-Casado G, Sanchez-Monge R, Gomez L, Castañera P, Salcedo G: The three inhibitor types from wheat endosperm are differentially active against _-amylases of lepidopteran pests. Entomol Exp Appl 66: 47–52 (1993).
Hines ME, Osuala CI, Nielsen SS: Isolation and partial characterization of a soybean cystatin cysteine proteinase inhibitor of Coleopteran digestive proteolytic activity. J Agric Food Chem 39: 1515–1520 (1991).
Kondo H, Abe K, Arai S: Immunoassay of oryzacystatin occurring in rice seeds during maturation and germination. Agric Biol Chem 53: 2949–2954 (1989).
Kondo H, Abe K, Nishimura I, Watanabe H, Emori Y, Arai S: Two distinct cystatin species in rice seeds with different specificities against cysteine proteinases. J Biol Chem 265: 15832–15837 (1990).
Kouzuma Y, Kawano K, Kimura M, Yamasaki N, Kadowaki T, Yamamoto K: Purification, characterization and sequencing of two cysteine proteinase inhibitors Sca and Scb from sun-flower (Helianthus aunuus) seeds. J Biochem 119: 1106–1113 (1996).
Kuroda M, Ishimoto M, Suzuki K, Kondo H, Abe K, Kitamura K, Arai S: Oryzocystatins exhibit growth-inhibitory and lethal effects on different species of bean insect pests Callosobruchus chinensis (Coleoptera) and Riptortus clavatus (Hemiptera). Biosci Biotechnol Biochem 60: 209–212 (1996).
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 (1970).
Leple JC, Bonade-Bottino M, Augustin S, Pilate G, Le Tan VD, Delplanque A, Cornu D, Jonanin L: Toxicity of Chrysomela tremulae (Coleoptera: Chrysomelidae) of transgenic poplars expressing a cysteine proteinase inhibitor. Mol Breed 1: 319–328 (1995).
Liang C, Brookhart G, Feng GH, Reeck GR, Kramer KJ: Inhibition of digestive proteinases of stored grain coleoptera by oryzacystatin, a cysteine proteinase inhibitor from rice seed. FEBS Lett 278: 139–142 (1991).
Misaka T, Kuroda M, Iwabuchi K, Abe K, Arai S: Soyacystatin, a novel cysteine proteinase inhibitor in soybean is distinct in protein structure and gene organization from other cystatins of animal and plant origin. Eur J Biochem 240: 609–614 (1996).
Ojima A, Shiota H, Higashi K, Kamada H, Shimma Y, Wada M, Satoh S: An extracellular insoluble inhibitor of cysteine proteinases in cell cultures and seeds of carrot. Plant Mol Biol 34: 99–109 (1997).
Robinson C, Kalsheker NA, Srinivasan N, King CM, Garrod DR, Thompson PJ, Stewart GA: On the potential significance of the enzymatic activity of mite allergens to immunogenicity. Clues to structure and function revealed by molecular characterization. Clin Exp Allergy 27: 10–21 (1997).
Salmia MA: Inhibitors of endogenous proteinases in Scots pine seeds: fractionation and activity changes during germination. Physiol Plant 48: 266–270 (1980).
Smith PK, Krohn RI, Hermason GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NW, Olson BJ, Klenk DC: Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85 (1985).
Turk V, Brzin J, Longer M, Ritonja A, Eropkin M, Borchart W, Maschleidt W: Protein inhibitors of cysteine proteinases. 3. Amino acid sequence of cystatin from egg withe. Hoppe-Seyler's Z Physiol Biochem 364: 1487–1496 (1983).
Turk B, Turk V, Turk D: Structural and functional aspects of papain-like cysteine proteinases and their protein inhibitors. Biol Chem 378: 141–150 (1997).
Urwin P, Atkinson HJ, Waller DA, PcPherson MJ: Engineered oryzacystatin-I expressed in transgenic hairy roots confers resistance to Globodera pallida. Plant J 8: 121–131 (1995).
Waldron C, Wegrich LM, Owens Merlo A, Walsh TA: Characterization of a genomic sequence coding for potato multicystatin, an eight-domain cysteine proteinase inhibitor. Plant Mol Biol 23: 801–812 (1993).
Zhao Y, Botella MA, Subramanian L, Niu X, Nielsen SS, Bressan RA, Hasegawa PM: Two wound-inducible soybean cysteine proteinase inhibitors have greater insect digestive proteinase inhibitory activities than a constitutive homolog. Plant Physiol 111: 1299–1306 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pernas, M., Sánchez-Monge, R., Gómez, L. et al. A chestnut seed cystatin differentially effective against cysteine proteinases from closely related pests. Plant Mol Biol 38, 1235–1242 (1998). https://doi.org/10.1023/A:1006154829118
Issue Date:
DOI: https://doi.org/10.1023/A:1006154829118