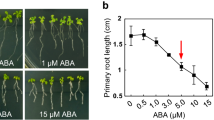
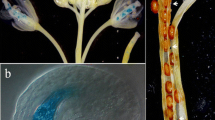
Abstract
Transgenic arabidopsis plants were isolated that contained a T-DNA construct in which the promoter of an auxin-inducible glutathione S-transferase (GST) gene from tobacco was fused to the kanamycin resistance (nptII) as well as to the β-glucuronidase (gusA) reporter gene. Subsequently, seeds were treated with EMS to obtain mutants in which both reporter gene fusions were up-regulated. Northern analysis showed that the mRNA level of a related, endogenous auxin-inducible GST gene of Arabidopsis was increased in some of these mutants as well. Two of the gup (GST up-regulated) mutants were characterized in more detail and roughly mapped. Both had epinastic cotyledons and leaves, a phenotype that turned out to be linked to the gup mutation.
Similar content being viewed by others
References
Abel S, Ballas N, Wong L-M, Theologis A: DNA elements responsive to auxin. BioEssays 18: 647–654 (1996).
Bernatzky R, Tanksley SD: Genetics of actin-related sequences in tomato. Theor Appl Genet 72: 314–321 (1986).
Bilang J, Macdonald H, King PJ, Sturm A: A soluble auxinbinding protein from Hyoscyamus muticus is a glutathione S-transferase. Plant Physiol 102: 29–34 (1993).
Bilang J, Sturm A: Cloning and characterization of a glutathione S-transferase that can be photolabeled with 5-azidoindole-3-acetic acid. Plant Physiol 109: 253–260 (1995).
Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inzé D: Superroot, a recessive mutation in arabidopsis, confers auxin overproduction. Plant Cell 7: 1405–1419 (1995).
Boot KJM, van der Zaal EJ, Velterop J, Quint A, Mennes AM, Hooykaas PJJ, Libbenga KR: Further characterization of expression of auxin-induced genes in tobacco (Nicotiana tabacum) cell-suspension cultures. Plant Physiol 102: 513–520(1993).
Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X: A mutation in arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 8: 1845–1857 (1994).
Brusslan JA, Tobin EM: Isolation of new promoter-mediated co-suppressed lines in Arabidopsis thaliana. Plant Mol Biol 27: 809–813 (1995).
Cao H, Bowling SA, Gordon AS, Dong X: Characterization of an arabidopsis mutant that is nonresponsive to inducers of systemic aquired resistance. Plant Cell 6: 1583–1592 (1994).
DeSouza L, King PJ: Mutants of Nicotiana plumbaginifolia with increased sensitivity to auxin. Mol Gen Genet 231: 65–75 (1991).
Droog F: Plant glutathione S-transferases, a tale of theta and tau. J Plant Growth Regul 16: 95–107 (1997).
Droog FNJ, Spek A, van der Kooy A, de Ruyter A, Hoge H, Libbenga KR, Hooykaas PJJ, van der Zaal EJ: Promoter analysis of the auxin-regulated tobacco glutathione S-transferase genes Nt103–1 and Nt103–35. Plant Mol Biol 29: 413–429 (1995).
Fabri CO, Schäffner AR: An Arabidopsis thaliana RFLP mapping set to localize mutations to chromosomal regions. Plant J 5: 149–156 (1994).
Feinberg AP, Vogelstein B: A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13 (1983).
Gamborg OL, Miller RA, Ojima K: Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158 (1968).
Goddijn OJM, Lindsey K, van der Lee FM, Klap JC, Sijmons PC: Differential gene expression in nematode-induced feeding structures of transgenic plants harbouring promoter-gusA fusion constructs. Plant J 4: 863–873 (1993).
Hagen G: The control of gene expression by auxin. In: Davies PJ (ed) Plant Hormones, pp. 228–245. Kluwer Academic Publishers, Dordrecht, Netherlands (1995).
Hobbie L, Estelle M: Genetic approaches to auxin action. Plant Cell Environ 17: 525–540 (1994).
Hood EE, Gelvin SB, Melchers LS, Hoekema A: New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2: 208–218 (1993).
Hooykaas PJJ, Klapwijk PM, Nuti MP, Schilperoort RA, Rörsch A: Transfer of the Agrobacterium tumefaciens Ti plasmid to avirulent agrobacteria and to Rhizobium ex planta. Gen Microbiol 98: 477–484 (1977).
Ishitani M, Xiong L, Stevenson B, Zhu J-K: Genetic analysis of osmotic and cold stress signal transduction in arabidopsis: interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell 9: 1935–1949 (1997).
Jefferson RA, Kavanagh TA, Bevan MW: GUS fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 (1987)
Jones AM, Prasad P V: Auxin-binding proteins and their possible reles in auxin-mediated plant cell growth. BioEssays 14: 43–47 (1992).
Karlin-Neumann GA, Brusslan JA, Tobin EM: Phytochrome control of the tms2 gene in transgenic arabidopsis: a stratagy for selecting mutants in the signal transduction pathway. Plant Cell 3: 573–582 (1991).
King JJ, Stimart DP, Fisher RH, Bleecker AB: A mutation altering auxin homeostasis and plant morphology in arabidopsis. Plant Cell 7: 2023–2037 (1995).
Klee HJ, Horsch RB, Hinchee MA, Hein MB, Hoffmann NL: The effects of overproduction of two Agrobacterium tumefaciens T-DNA auxin biosynthetic gene products in transgenic petunia plants. Genes Devel 1: 86–96 (1987).
Konieczny A, Ausubel FM: A procedure for mapping arabidopsis mutations using co-dominant ecotype-specific PCRbased markers. Plant J 4: 403–410 (1993).
Leyser HMO, Pickett FB, Dharmasiri S, Estelle M: Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J 10: 403–413 (1996).
Leyser O: Auxin: lessons from a mutant weed. Physiol Plant 100: 407–414 (1997).
Marsh JL, Erfle M, Wykes EJ: The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene 32: 481–485 (1984).
Mattanovich D, Rüker F, da Câmara Machado A, Laimer M, Regner F, Steinkellner H, Himmler G, Katinger H: Ef-ficient transformation of Agrobacterium spp. by electroporation. Nucl Acids Res 17: 6747 (1989).
Miao Z-H, Liu X, Lam E: TGA3 is a distinct member of the TGA family of bZIP transcription factors in Arabidopsis thaliana. Plant Mol Biol 25: 1–11 (1994).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Napier RM, Venis RA: Auxin action and auxin binding proteins. New Phytol 129: 167–201 (1995).
Neuhaus G, Neuhaus-Url G, Katagiri F, Seipel K, Chua N-H: Tissue-specific expression of as-1 in transgenic tobacco. Plant Cell 6: 827–834 (1994).
Neuhaus-Url G, Neuhaus G: The use of the nonradioactive digoxigenin chemiluminescent technology for plant genomic Southern blot hybridization: a comparison with radioactivity. Transgenic Res 2: 115–120 (1993).
Romano CP, Cooper ML, Klee HJ: Uncoupling auxin and ethylene effects in transgenic tobacco and Arabidopsis plants. Plant Cell 5: 181–189 (1993).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sitbon F, Hennion S, Sundberg B, Little CHA, Olsson O, Sandberg G: Transgenic tobacco plants coexpressing the Agrobacterium tumefaciens iaaM and iaaH genes display altered growth and indoleacetic acid metabolism. Plant Physiol 99: 1062–1069 (1992).
Sitbon F, Perrot-Rechenmann C: Expression of auxinregulated genes. Physiol Plant 100: 443–455 (1997).
Susek RE, Ausubel FM, Chory J: Signal transduction mutants of arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 74: 787–799 (1993).
Valvekens D, Van Montagu M, Van Lijsebettens M: Agrobacterium tumefaciens mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA 85: 5536–5540 (1988).
van den Elzen PJM, Townsend J, Lee KY, Bedbrook JR: A chimaeric hygromycin resistance gene as a selectable marker in plant cells. Plant Mol Biol 5: 299–302 (1985).
van der Kop DAM, Droog FNJ, van der Zaal BJ, Hooykaas PJJ: Expression of an auxin-inducible promoter of tobacco in Arabidopsis thaliana. Plant Growth Regul 18: 7–14 (1996).
van der Kop DAM, Schuyer M, Scheres B, van der Zaal BJ, Hooykaas PJJ: Isolation and characterization of an auxin-inducible glutathione S-transferase gene of Arabidopsis thaliana. Plant Mol Biol 30: 839–844 (1996).
van der Zaal BJ, Droog FNJ, Pieterse FJ, Hooykaas PJJ: Auxin-sensitive elements from promoters of tobacco gst genes and a consensus as-1-like element differ only in relative strength. Plant Physiol 110: 79–88 (1996).
van der Zaal EJ, Droog FNJ, Boot CJM, Hensgens LAM, Hoge JHC, Schilperoort RA, Libbenga KR: Promoters of auxin-induced genes from tobacco can lead to auxin-inducible and root tip-specific expression. Plant Mol Biol 16: 983–998 (1991).
van Slogteren GMS, Hoge JHC, Hooykaas PJJ, Schilperoort RA: Clonal analysis of heterogenous crown gall tumour tissues induced by wild-type and shooter mutant strains of Agrobacterium tumefaciens: expression of T-DNA genes. Plant Mol Biol 2: 321–333 (1983).
Zettl R, Schell J, Palme K: Photoaffinity labeling of Arabidopsis thaliana plasma membrane vesicles by 5-azido–[7–3H]indole-3-acetic acid: identification of a glutathione S-transferase. Proc Natl Acad Sci USA 91: 689–693 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van der Kop, D.A., Schuyer, M., Pinas, J.E. et al. Selection of Arabidopsis mutants overexpressing genes driven by the promoter of an auxin-inducible glutathione S-transferase gene. Plant Mol Biol 39, 979–990 (1999). https://doi.org/10.1023/A:1006129426712
Issue Date:
DOI: https://doi.org/10.1023/A:1006129426712