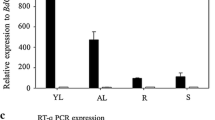
Abstract
The transcription factors VP1 (Viviparous-1), EmBP-1 (Em-binding protein 1) and OSBZ8, originally cloned and analysed in various monocot species, have been implicated in the regulation of the Lea (late embryogenesis-abundant) group 1 genes. We have investigated the modulation of the levels of these mRNAs in barley during embryogenesis, and in developing embryos subjected to various kinds of osmotic stress. The accumulation of mRNA for VP1 and EmBP-1 transcription factors, using cDNAs cloned from barley, starts at 10 and 15 days after anthesis, respectively, whereas Lea B19 mRNA levels are very low or undetectable until 25 days after anthesis during normal development. The EmBP-1 mRNA is predominantly induced in mannitol-stressed immature embryos. Vp1 mRNA was not significantly modulated by ABA, salt or mannitol. Inhibition of ABA biosynthesis by norflurazon showed that the induction of both Vp1 and EmBP-1 mRNAs was ABA-independent. In embryo-derived suspension-cultured cells, neither of the two transcripts would be induced by ABA or osmotic stress, although both OSBZ8 and one member of the Lea B19 family was up-regulated by ABA. Electrophoretic mobility shift assays using a Lea B19.1 probe with an ABRE (abscisic acid-responsive element) similar to that which binds EmBP-1 and OSBZ8 in the wheat and rice Em promoters show that the binding activity is increased by ABA and osmotic stress. Taken together, these data show that both VP1 and EmBP-1 are involved in embryo-specific signal transduction pathways, that they are differentially regulated at the mRNA level, and that EmBP-1 can be induced by osmotic stress independently of any increase in endogenous ABA. The difference in mRNA regulation patterns of OSBZ8 and EmBP-1 may suggest that they are involved in different signal transduction pathways in connection with osmotic stress/ABA regulation of Lea genes.
Similar content being viewed by others
References
Aalen RB, Ferstad H-GO, Linnestad C, Olsen O-A: Transcripts encoding an oleosin and a dormancy related protein are present both in the aleurone layer and in the embryo of developing barley (Hordeum vulgare L.) seeds. Plant J 5: 385–396 (1994).
Bobb AJ, Eiben HG, Bustos MM: PvAlf, an embryo-specific acidic transcriptional activator enhances gene expression from phaseolin and phytohemagglutinin promoters. Plant J 8: 331–343 (1995).
Bostock RM, Quatrano RS: Regulation of Em gene expression in rice. Plant Physiol 98: 1356–1363 (1992).
Delauney AJ, Verma DPS: Proline biosynthesis and osmoregulation in plants. Plant J 4: 215–223 (1993).
Devos KM, Atkinson MD, Chinoy CN, Guiltinan MJ, Quatrano RS, Gale MD: Chromosomal location and variability in wheat, barley and rye of awheat gene encoding a bZIP protein (EmBP-1). Theor Appl Genet 82: 665–667 (1991).
Espelund M, Stacy RAP, Jakobsen KS: A simple method for generating single-stranded DNA probes labeled to high activities. Nucl Acids Res 18: 6157–6158 (1990).
Espelund M, Jakobsen KS: Cloning and direct sequencing of plant promoters using primer-adapter mediated PCR on DNA coupled to a magnetic solid phase. BioTechniques 13: 74–81 (1992).
Espelund M, Sæbøe-Larssen S, Hughes DW, Galau GA, Larsen F, Jakobsen KS: Late embryogenesis-abundant genes encoding proteins with different numbers of hydrophilic repeats are regulated differentially by abscisic acid and osmotic stress. Plant J 2: 241–252 (1992).
Espelund M, De Bedout JA, Outlaw WH Jr, Jakobsen KS: Environmental and hormonal regulation of barley lateembryogenesis-abundant (Lea) mRNAs is via different signal transduction pathways. Plant Cell Environ 18: 943–949 (1995).
Finkelstein RR: Abscisic acid-insensitive mutations provide evidence for stage-specific signal pathways regulating expression of an Arabidopsis late embryogenesis-abundant (lea) gene. Mol Gen Genet 238: 401–408 (1993).
Galau GA, Hughes DW, Dure L III: Abscisic acid induction of cloned cotton late embryogenesis-abundant (Lea) mRNAs. Plant Mol Biol 7: 155–170 (1986).
Galau GA, Jakobsen KS, Hughes DW: The controls of late dicot embryogenesis and early germination. Physiol Plant 81: 280–288 (1991).
Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM: Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 4: 1251–1261 (1992).
Guiltinan MJ, Marcotte WR Jr, Quatrano RS: A plant leucine zipper protein that recognizes an abscisic acid response element. Science 250: 267–271 (1990).
Guiltinan MJ, Miller L: Molecular characterization of the DNA-binding and dimerization domains of the bZIP transcription factor, EmBP-1. Plant Mol Biol 26: 1041–1053 (1994).
Hattori T, Vasil V, Rosenkrans L, Hannah LC, McCarty DR, Vasil IK: The Vivoparous-1 gene and abscisic acid activate the C1 regulatory gene for anthocyanin biosynthesis during seed maturation in maize. Genes Devel 6: 609–618 (1992).
Hattori T, Terada T, Hamasuna ST: Sequence and functional analyses of the rice gene homologous to the maize Vp1. Plant Mol Biol 24: 805–810 (1994).
Hattori T, Terada T, Hamasuna ST: Regulation of the Osem gene by abscisic acid and the transcriptional activator VP1: analysis of cis-acting promoter elements required for regulation by abscisic acid and VP1. Plant J 7: 913–925 (1995).
Hetherington AM, Quatrano RS: Mechanisms of action of abscisic acid at the cellular level. NewPhytol 119: 9–32 (1991).
Hill A, Nantel A, Rock CD, Quatrano RS: A conserved domain of the viviparous-1 gene product enhances the DNA binding activity of the bZIP protein EmBP-1 and other transcription factors. J Biol Chem 271: 3366–3374 (1996).
Hollung K, Espelund M, Jakobsen KS: Another Lea B19 gene (Group 1 Lea) from barley containing a single 20 amino acid hydrophilic motif. Plant Mol Biol 25: 559–564 (1994).
Hollung K, Gabrielsen OS, Jakobsen KS: Enrichment of DNA binding proteins from crude tissue for electrophoretic mobility shift assay using magnetic phospho cellulose particles. Nucl Acids Res 22: 3261–3262 (1994).
Hughes DW, Galau GA: Developmental and environmental induction of Lea and LeaA mRNAs and the postabscission program during embryo culture. Plant Cell 3: 605–618 (1991).
Hultman T, Ståhl S, Hornes E, Uhlèn M: Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucl Acids Res 17: 4937–4946 (1989).
Jähne A, Lazzeri PA, Jäger-Gussen M, Lörz H: Plant regeneration from embryogenic cell suspensions derived from anther cultures of barley (Hordeum vulgare L.) Theor Appl Genet 82: 74–80 (1991).
Jakobsen KS, Breivold E, Hornes E: Purification of mRNA directly from crude plant tissue in 15 minutes using magnetic oligo dT microspheres. Nucl Acids Res 18: 3669 (1990).
Jakobsen KS, Hughes DW, Galau GA: Simultaneous induction of postabscission and germination mRNAs in cultured dicot embryos. Planta 192: 384–394 (1994).
Jakobsen KS, Haugen M, Sæbøe-Larssen S, Hollung K, Espelund M, Hornes E: DirectmRNAisolation usingmagnetic oligo dT beads: A protocol for all types of cell cultures, animal and plant tissues. In: Uhlen M, Hornes E, Olsvik Ø (eds) Advances in Biomagnetic Separation, pp. 61–71. Eaton Publishers, MA (1994).
Klemsdal SS, Kvaale A, Olsen O-A: Effects of the barley mutants Risø 1508 and 527 high lysine genes on the cellular development of the endosperm. Physiol Plant 67: 453–459 (1986).
McCarty DR, Carson CB, Stinard PS, Robertson DS: Molecular analysis of vivoparous-1: an abscisic acid-insensitive mutant of maize. Plant Cell 1: 523–532 (1989).
McCarty DR, Hattori T, Carson CB, Vasil V, Lazar M, Vasil IK: The Viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell 66: 895–905 (1991).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Nakagawa H, Ohmiya K, Hattori T: A rice bZIP protein, designated OSB28, is rapidly induced by abscisic acid. Plant J 9: 217–227 (1996).
Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J: Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6: 1567–1582 (1994).
Prat D, Fathi-Ettai RA: Variation in organic and mineral components in young Eucalyptus seedlings under saline stress. Physiol Plant 79: 479–486 (1990).
Snustad DP, Haas NA, Kopczak SD, Silflow CD: The small genome of Arabidopsis contains at least nine expressed β-tubulin genes. Plant Cell 4: 549–556 (1992).
Stacy RAP, Espelund M, Soebøe-Larssen S, Hollung K, Helliesen E, Jakobsen KS: Evolution of the Group 1 late embryogenesis abundant (Lea) genes: analysis of the Lea B19 gene family in barley. Plant Mol Biol 28: 1039–1054 (1995).
Taylor JE, Renwick KF, Webb AAR, McAinsh MR, Furini A, Bartels D, Quatrano RS, Marcotte Jr WR, Hetherington AM: ABA-regulated promoter activity in stomatal guard cells. Plant J 7: 129–134 (1995).
Vasil IK, Vasil V: Advances in cereal protoplast research. Physiol Plant 85: 279–283 (1992).
Vasil V, Marcotte WT Jr, Rosenkrans L, Cocciolone SM, Vasil IK, Quatrano RS, McCarty DR: Overlap of Viviparous1 (VP1) and abscisic acid response elements in the Empromoter: G-box elements are sufficient but not necessary for VP1 transactivation. Plant Cell 7: 1511–1518 (1995).
Vernon DM, Ostrem JA, Bohnert HJ: Stress perception and response in a facultative halophyte: the regulation of salinityinduced genes in Mesembryanthemum crystallinum. Plant Cell Environ 16: 437–444 (1993).
Williams JD, Scandalios JG: Differential response of maize catalases to abscisic acid: Vp1 transcriptional activator is not required for abscisic acid-regulated Cat1 expression. Proc Natl Acad Sci USA 89: 8842–8846 (1992).
Yamaguchi-Shinozaki K, Shinozaki K: A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6: 251–264 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hollung, K., Espelund, M., Schou, K. et al. Developmental, stress and ABA modulation of mRNA levels for bZip transcription factors and Vp1 in barley embryos and embryo-derived suspension cultures. Plant Mol Biol 35, 561–571 (1997). https://doi.org/10.1023/A:1005815017718
Issue Date:
DOI: https://doi.org/10.1023/A:1005815017718