Abstract
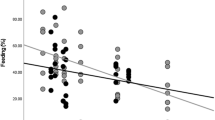
The ecological constraints model proposes that an increase in group size will increase intragroup feeding competition and thereby constrain group size. Although this model has received wide acceptance, tests of it are based only on a few studies of species that have similar ecological requirements and social organizations, and there are reasons to question the widespread acceptance of the assumptions underpinning it. Via a 2-year study, we explored determinants of group size in species that feed on markedly different types of foods: the folivorous red colobus (Procolobus pennantii) and the frugivorous/insectivorous red-tailed guenon (Cercopithecus ascanius). We established 4 study sites approximately 15 km apart in Kibale National Park, Uganda, to examine the relationship between average group size and food availability. In both species, we quantified interdemic variation in diet, density of food trees, rate of travel, and group size. Red colobus at all sites relied heavily on leaf resources (75.5%–86.9%), but fruit (6.4%–13.9%) and flowers (2.0%–13.9%) were important in some populations. In general, red-tailed guenons fed on fruit (35.7%–59.7%), insects (14.5%–17.6%), and young leaves (12.2%–32.8%), but the amount of time allocated to these foods varied among sites. Average monthly density of trees bearing food items ranged among sites from 45 to 79 trees/ha for red colobus and from 19.6 to 67.3 trees/ha for red-tailed guenons. For both species, rate of travel was similar among sites, with one exception for red colobus. Average red colobus group size varied among sites from 14 to 40 (28 groups counted). Red-tailed guenon group size varied among sites from 11 to 24 (16 groups counted). As predicted by the ecological constraints model, group size increased with food tree density across sites for both species.
Similar content being viewed by others
REFERENCES
Altmann, S. (1974). Baboons, space, time, and energy. Amer. Zool. 14: 221–248.
Barton, R. A., Byrne, R. W., and Whiten, A. (1996). Ecology, feeding competition and social structure in baboons. Behav. Ecol. Sociobiol. 38: 321–329.
Bradbury, J. W., and Vehrencamp, S. L. (1976). Social organization and foraging in emballonurid bats. II. A model for the determination of group size. Behav. Ecol. Sociobiol. 1: 383–404.
Butynski, T. M. (1990). Comparative ecology of blue monkeys (Cercopithecus mitis) in highand low-density subpopulations. Ecol. Monogr. 60: 1–26.
Byrne, R. W., Whiten, A., Henzi, S. P., and McCulloch, F. M. (1993). Nutritional constraints on mountain baboons (Papio ursinus): Implications for baboon socioecology. Behav. Ecol. Sociobiol. 33: 233–246.
Chapman, C. A. (1988a). Patch use and patch depletion by the spider and howling monkeys of Santa Rosa National Park, Costa Rica. Behaviour 105: 88–116.
Chapman, C. A. (1988b). Patterns of foraging and range use by three species of neotropical primates. Primates 29: 177–194.
Chapman, C. A. (1989). Ecological constraints on group size in three species of neotropical primates. Folia Primatol. 73: 1–9.
Chapman, C. A. (1990a). Ecological constraints on group size in three species of neotropical primates. Folia Primatol. 73: 1–9.
Chapman, C. A. (1990b). Association patterns of spider monkeys: The influence of ecology and sex on social organization. Behav. Ecol. Sociobiol. 26: 409–414.
Chapman, C. A., and Chapman, L. J. (2000). Determinants of group size in social primates: The importance of travel costs. In Boinski, S. and Garber, P. (eds.), Group Movement in Social Primates and Other Animals: Patterns, Processes, and Cognitive Implications. University of Chicago Press, Chicago, pp. 24–42.
Chapman, C. A. and Chapman L. J. (1999). Implications of small scale variation in ecological conditions for the diet and density of red colobus monkeys. Primate 40: 215–232.
Chapman, C. A. and Chapman L. J. (1999). Forest regeneration in logged and unlogged forests of Kibale National Park, Uganda. Biotropica 29: 396–412.
Chapman, C. A., Chapman, L. J., Wrangham, R, Isabirye-Basuta, G., and Ben-David, K. (1997). Spatial and temporal variability in the structure of a tropical forest. Afr. J. Ecol. 35: 287–302.
Chapman, C. A., White, F., and Wrangham, R. W. (1994). Party size in chimpanzees and bonobos: A reevaluation of theory based on two similarly forested sites. In McGrew, W. C., Marchant, L. F., and Nishida T. (eds.), Chimpanzee Cultures, Harvard University Press, Cambridge, pp. 45–57.
Chapman, C. A., Wrangham, R. W., and Chapman, L. J. (1995). Ecological constraints on group size: An analysis of spider monkey and chimpanzee subgroups. Behav. Ecol. Sociobiol. 36: 59–70.
Chapman, C. A., Wrangham, R. W., Chapman, L. J., Kennard, D. K., and Zanne, A. E. (1999). Fruit and flower phenology at two sites in Kibale National Park, Uganda. J. Trop. Ecol. 15: 189–211.
Charnov, E. L. (1976). Optimal foraging: The marginal value theorem. Theor. Pop. Biol. 9: 129–136.
Cheney, D. L. (1992). Intragroup cohesion and intergroup hostility: The relation between grooming distributions and intergroup competition among female primates. Behav. Ecol. 3: 334–345.
Chism, J., and Rowell, T. E. (1988). The natural history of patas monkeys. In Gautier-Hion, A., Bourliere, F., Gautier, J.-P., and Kingdon, J. (eds.), A Primate Radiation: Evolution of the African Guenons. Cambridge University Press, Cambridge, pp. 412–438.
Clutton-Brock, T. T., and Harvey, P. H. (1977). Primate ecology and social organization. J. Zool., London 183: 1–39.
Dittus, W. P. J. (1979). The evolution of behaviour regulating density and age-specific sex rations in a primate population. Behaviour 69: 265–302.
Elgar, M. A. (1986). House sparrows establish foraging flocks by giving chirrup calls if the resources are divisible. Anim. Behav. 34: 169–174.
Howard, P. C. (1991). Nature Conservation in Uganda' Tropical Forest Reserves. IUCN, Gland, Switzerland.
Isbell, L. A. (1983). Daily ranging behavior of red colobus (Colobus badius tephrosceles) in Kibale Forest, Uganda. Folia Primatol. 41: 34–48.
Isbell, L. A. (1991). Contest and scramble competition: patterns of female aggression and ranging behaviour among primates. Behav. Ecol. 2: 143–155.
Isbell, L. A., Pruetz, J. D., and Young, T. P. (1998). Movements of vervets (Cercopithecus aethiops) and patas monkeys (Erythrocebus patas) as estimators of food resource size, density, and distribution. Behav. Ecol. Sociobiol. 42: 123–133.
Janson, C. H. (1985). Aggressive competition and individual food consumption in wild brown capuchin monkeys (Cebus apella). Behav. Ecol. Sociobiol. 18: 125–138.
Janson, C. H. (1988). Intra-specific food competition and primate social structure: A synthesis. Behaviour 105: 1–17.
Janson, C. H., and Goldsmith, M. L. (1995). Predicting group size in primates: Foraging costs and predation risks. Behav. Ecol. 6: 326–336.
Kingston, B. (1967). Working Plan for Kibale and Itwara Central Forest Reserves. Entebbe, Uganda, Uganda Forest Department.
MacDonald, D. W. (1979). The flexible social system of the golden jackal, Canis aureus. Behav. Ecol. Sociobiol. 5: 17–38.
McDonald, D. W. (1983). The ecology of carnivore social behaviour. Nature 301: 379–384.
Milton, K. (1984). Habitat, diet, and activity patterns of free-ranging woolly spider monkeys (Brachyteles arachnoides E. Geoffroyi 1806). Int. J. Primatol. 5: 491–514.
Nicholson, A. J. (1954). An outline of the dynamics of animal populations. Aust. J. Zool. 2: 9–65.
Olupot, W., Chapman, C. A., Brown, C. H., and Waser, P. M. (1994). Mangabey (Cercocebus albigena) population density, group size, and ranging: A twenty-year comparison. Amer. J. Primatol. 32: 197–205.
Osmaston H. A. (1959). Working Plan for the Kibale and Itwara Forests. Uganda Forest Dept. Entebbe, 60 p.
Pulliam H. R., and Caraco, T. (1984). Living in groups: is there an optimal group size? In Krebs, J. R., and Davis, N. (eds.), Behavioural Ecology, Sinauer, Sunderland, pp. 122–147.
Schaik, C. P. van. (1989). The ecology of social relationships amongst female primates. In Standen, V. and Foley, R. A. (eds.), Comparative Socioecology, Blackwell Press, Cambridge, pp. 195–218.
Schaik C. P. van, and M. A. van Noordwijk. (1988). Scramble and contest in feeding competition among female long-tailed macaques (Macaca fascicularis). Behaviour 105: 77–98.
Schaik C. P. van, M. A. van Noordwijk, R.J. Boer, and I. Den Tonkelaar. (1983). The effect of group size on time budgets and social behaviour in wild long-tailed macaques (Macaca fascicularis). Behav. Ecol. Sociobiol. 13: 173–181.
Skorupa, J. P. (1988). The effect of selective timber harvesting on rain-forest primates in Kibale Forest, Uganda. Unpublished Ph.D. Dissertation, University of California, Davis, California, USA.
Stephens, D. W., and Krebs, J. R. (1986). Foraging Theory. Princeton University Press, Princeton.
Strier, K. B. (1989). Effects of patch size on feeding associations in muriquis (Brachyteles arachnoides). Folia Primatol. 52: 70–77.
Struhsaker, T. T. (1967). Ecology of vervet monkeys (Cercopithecus aethiops) in the Masai-Amboseli Game Reserve, Kenya. Ecology 48: 891–904.
Struhsaker, T. T. (1975). The red colobus monkey. University of Chicago Press, Chicago.
Struhsaker, T. T. (1980). Comparison of the behaviour and ecology of red colobus and redtail monkeys in the Kibale Forest, Uganda. Afr. J. Ecol. 18: 33–51.
Struhsaker, T. T. (1997). Ecology of an African Rain Forest: Logging in Kibale and the Conflict between Conservation and Exploitation. Gainesville, Florida, The University Presses of Florida.
Struhsaker, T. T., and Leland, L. (1987). Colobines: Infanticide by adult males. In Smuts, B., Cheney, D. L., Seyfarth, R. M., Wrangham, R. W., and Struhsaker, T. T. (eds.), Primate Societies, University of Chicago Press, Chicago, pp. 83–97.
Struhsaker, T. T., and Leland, L. (1988). Group fission in red-tail monkeys (Cercopithecus ascanius) in the Kibale Forest, Uganda. In Gautier-Hion, A., Bourliere, F., Gautier, J.-P., and Kingdon, J. A Primate Radiation: Evolution of the African Guenons. Cambridge University Press, Cambridge, pp. 364–388.
Symington, M. M. (1987). Ecological and social correlates of party size in the black spider monkey. Ateles paniscus chamek. Ph.D. Dissertation, Princeton, New Jersey.
Symington, M. M. (1988a). Food competition and foraging party size in the black spider monkey (Ateles paniscus chamek). Behaviour 105: 117–134.
Symington, M. M. (1988b). Demography, ranging patterns and activity budgets of black spider monkeys (Ateles paniscus chamek) in the Manu National Park, Peru. Amer. J. Primatol. 15: 45–67.
Symington, M. M. (1990). Fission-fusion social organization in Ateles and Pan. Int. J. Primatol. 11: 47–61.
Terborgh, J. (1983). Five New World Primates. Princeton University Press, Princeton.
Terborgh, J., and Janson, C. H. (1986). The socioecology of primate groups. Ann. Rev. Ecol. Syst. 17: 111–135.
Waser, P. M. (1974). Inter-group interactions in a forest monkey the mangabey Cercocebus albigena. Ph.D. Dissertation, Rockefeller University, New York.
Waser, P. M. (1981). Sociality or territorial defense? The influence of resource renewal. Behav. Ecol. Sociobiol. 8: 231–237.
White, F. J., and Wrangham, R. W. (1988). Feeding competition and patch size in the chimpanzee species Pan paniscus and Pan troglodytes. Behaviour 105: 148–164.
Whitten, P. (1983). Diet and dominance among female vervet monkeys (Cercopithecus aethiops). Amer. J. Primatol. 5: 139–159.
Wrangham, R. W., Gittleman, J. L., and Chapman, C. A. (1993). Constraints on group size in primates and carnivores: population density and day-range as assays of exploitation competition. Behav. Ecol. Sociobiol. 32: 199–210.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chapman, C.A., Chapman, L.J. Constraints on Group Size in Red Colobus and Red-tailed Guenons: Examining the Generality of the Ecological Constraints Model. International Journal of Primatology 21, 565–585 (2000). https://doi.org/10.1023/A:1005557002854
Issue Date:
DOI: https://doi.org/10.1023/A:1005557002854