Abstract
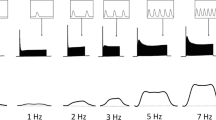
Isometric tension responses to rapid temperature jumps (T-jumps) of 2–6°C were examined in skinned muscle fibre bundles isolated from papillary muscles of the rat heart. T-jumps were induced by an infra-red laser pulse (wave length 1.32 μm, pulse duration 0.2 ms) obtained from a Nd-YAG laser, which heated the fibres and bathing buffer solution in a 50 μl trough; the increased temperature by laser pulse was clamped at the high temperature by a Peltier system (see Ranatunga, 1996). In maximally Ca2+-activated (pCa ca. 4.5) fibres, the relationship between tension and temperature was non-linear, the increase of active tension with temperature being more pronounced at lower temperatures (below ca. 20°C). A T-jump at any temperature (range 3–35°C) induced an initial step decrease of tension of variable amplitude (Phase 1), probably due to thermal expansion, and it was followed by a tension transient which resulted in a net rise of tension above the pre-T-jump level. The rate of net rise of tension (Phase 2b or endothermic force generation) was 7–10/s at ca. 12°C and its Q10 was 6.3 (below 25°C). In cases where the step decrease of tension in Phase 1 was prominent, an initial quick tension recovery phase (Phase 2a, 70–100/s at 12°C) that did not contribute to a rise of tension above the pre-T-jump level, was also seen. This phase (Phase 2a) appeared to be similar to the quick tension recovery induced by a small length release and its rate increased with temperature with a Q10 of 1.8. In some cases where Phase 2a was present, a slower tension rise (Phase 3) was seen; its rate (ca. 5/s) was temperature-insensitive. The results show that the rate of endothermic force generation in cardiac fibres is clearly different from that of either fast-twitch or slow-twitch mammalian skeletal muscle fibres; implication of such fibre type-specific differences is discussed in relation to the difficulty in identifying the biochemical step underlying endothermic cross-bridge force generation.
Similar content being viewed by others
References
Bershitsky SY and Tsaturyan AK (1989) Effect of joule temperature jump on tension and stiffness of skinned rabbit muscle fibers. Biophys J 56: 809–816.
Bershitsky SY and Tsaturyan AK (1992) Tension responses to joule temperature jump in skinned rabbit muscle fibers. J Physiol 447: 425–448.
Bershitsky SY, Tsaturyan AK, Bershitskaya ON, Mashanov GI, Brown P, Burns R and Ferenczi MA (1997) Muscle force is generated by myosin heads stereospecifically attached to action. Nature 388: 186–190.
Dantzig JA, Goldman YE, Millar NC, Lacktis J and Homsher E (1992) Reversal of the crossbridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol 451: 247–275.
Davis JS and Harrington WF (1987) Force generation by muscle fibers in rigor: a laser temperature-jump study. Proc Natl Acad Sci USA 84: 975–979.
Davis JS and Harrington WF (1993) A single order-disorder transition generates tension during the Huxley-Simmons phase-2 in muscle. Biophys J 65: 1886–1898.
Davis JS and Rodgers ME (1995a) Force generation and temperature-jump and length-jump tension transients in muscle fibers. Biophys J 68: 2032–2040.
Davis JS and Rodgers ME (1995b) Indirect coupling of phosphate release to de-novo tension generation during muscle contraction. Proc Natl Acad Sci USA 92: 10482–10486.
De Tombe PP and ter Keurs HEDJ (1990) Force and velocity of sarcomere shortening in trabeculae from rat heart. Circul Res 66: 1239–1254.
Ford LE, Huxley AF and Simmons RM (1977) Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol 269: 441–515.
Fortune NS, Geeves MA and Ranatunga KW (1989) Pressure sensitiv-ity of active tension in glycerinated rabbit psoas muscle fibres: effect of ADP and phosphate. J Muscle Res Cell Motil 10: 113–123.
Fortune NS, Geeves MA and Ranatunga KW (1991) Tension responses to rapid pressure release in glycerinated rabbit muscle fibers. Proc Natl Acad Sci USA 88: 7323–7327.
Goldman YE, McCray JA and Ranatunga KW (1987) Transient tension changes initiated by laser temperature jumps in rabbit muscle fibres. J Physiol 392: 71–95.
Harrison SM and Bers DM (1990) Modification of temperature dependence of myofilament Ca sensitivity by troponin C replace-ment. Am J Physiol 258: C282–C288.
Huxley AF and Simmons RM (1971) Proposed mechanism of force generation in striated muscle. Nature 233: 533–538.
Irving M, Lombardi V, Piazzesi G and Ferenczi MA (1992) Myosin head movements are synchronous with the elementary force-generating process in muscle. Nature 357: 156–158.
Irving M, Allen T St C, Sabido-David C, Craik JS, Brandmeirer B, Kendrick-Jones J, Corrie JET, Trentham DR and Goldman YE (1995) Tilting of the light-chain region of myosin during step length changes and active force generation in skeletal muscle. Nature 375: 688–691.
Millar NC and Holmsher E (1992) Kinetics of force generation and phosphate release in skinned rabbit soleus muscle fibers. Am J Physiol 262: C1239–C1245.
Ranatunga KW (1984) The force-velocity relation of rat fast-and slow-twitch muscles examined at different temperatures. J Physiol 351: 517–529.
Ranatunga KW (1994) Thermal stress and Ca-independent contractile activation in mammalian skeletal muscle fibers at high tempera-tures. Biophys J 66: 1531–1541.
Ranatunga KW (1996) Endothermic force generation in fast and slow mammalian (rabbit) muscle fibers. Biophys J 71: 1905–1913.
Ranatunga KW (1997) Laser temperature jump experiments on mammalian (rat) cardiac muscle fibers. J Physiol 504.P: 62P.
Wang G and Kawai M (1997) Force generation and phosphate release steps in skinned rabbit soleus slow-twitch muscle fibers. Biophys J 73: 878–894.
Zhao Y and Kawai M (1994) Kinetic and thermodynamic studies of the cross-bridge cycle in rabbit psoas muscle fibers. Biophys J 67: 1655–1688.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ranatunga, K.W. Endothermic force generation in skinned cardiac muscle from rat. J Muscle Res Cell Motil 20, 489–496 (1999). https://doi.org/10.1023/A:1005509731881
Issue Date:
DOI: https://doi.org/10.1023/A:1005509731881