Abstract
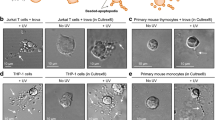
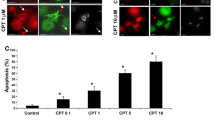
To investigate the early event of apoptosis, we monitored the morphological changes in the early stage of Fas-induced apoptosis in the human T-cell lymphoma cell line Jurkat, using confocal microscopy. Morphological changes in the nuclei were observed from 30 min after stimulation, and preceded the changes in the cytoskeleton. This kind of change was enhanced in the presence of EGTA but decreased in the presence of dihydrocytochalasin B, without any changes in caspase-3 activation. During the changes in shape of the cells, the actin cytoskeleton collapsed and shrank in the center. Even though nuclei also changed their shapes in apoptotic cells, they were partially TUNEL-negative, suggesting that they were not yet damaged at the DNA level. Our results suggest that, in the process of apoptosis in Jurkat cells, cell nuclei and cytoskeleton are changed first, then membrane blebbing and caspase-3 activation occur, and fragmentation of chromosomal DNA is last.
Similar content being viewed by others
References cited
Berridge MJ, Boothman MD, Lipp P (1998) Calcium-a life and death signal. Nature 395: 645-648.
Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D (1995) A novel protein that interacts with the death domain of Fas/APO-1 contains a sequence motif related to the death domain. J Biol Chem 270: 7795-7798.
Caulin C, Salvesen GS, Ohshima RG (1997) Caspase cleavage of keratin 18 and reorganization of intermediate filaments during cell apoptosis. J Cell Biol 138: 1379-1394.
Cerretti DP, Kozlosky CJ, Mosley BS, Nelson N, Ness KV, Greenstreet TA, March CJ, Kronenheim SR, Druck T, Canizzaro LA, Huebner K, Black RA (1992) Molecular cloning of the interleukin-1? converting enzyme. Science 256: 97-100.
Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM (1995) FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81: 505-512.
Enari M, Sakahira H, Yokoyama H, Okayama K, Iwamatsu A, Nagata S (1998) Acaspase-activated DNase that degradesDNAduring apoptosis, and inhibitor ICAD. Nature 391: 43-50.
Fernandes-Alnemri T, Litwack G, Alnemri ES (1994) CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1?-converting enzyme. J Biol Chem 269: 30761-30764.
Fesus L, Thomazy V, Falus A (1987) Induction and activation of tissue transglutaminase during programmed cell death. FEBS Lett 224: 104-108.
Hampton MB, Vanags DM, Pörn-Ares I, Orrenius S (1996) Involvement of extracellular calcium in phosphatidylserine exposure during apoptosis. FEBS Lett 399: 277-282.
Horino K, Nishiura H, Ohsako T, Shibuya Y, Hiraoka T, Kitamura N, Yamamoto T (1998) A monocyte chemotactic factor, S19 ribosomal protein dimer, in phagocytotic clearance of apoptotic cells. Lab Invest 78: 603-617.
Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S (1991) The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 66: 233-243.
Kamada S, Kusano H, Fujita H, Ohtsu M, Koya RC, Kuzumaki N, Tsujimoto Y (1998) A cloning method for caspase system: cloning of the antiapoptotic gene gelsolin. Proc Natl Acad Sci USA 95: 8532-8537.
Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT (1997) Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science 278: 294-298.
Lazebnik YA, Cole S, Cooke CA, Nelson WG, Earnshaw WC (1993) Nuclear events of apoptosis in vitro in cell-free mitotic extracts: a model system for analysis of the active phase of apoptosis. J Cell Biol 123: 7-22.
MashimaT, Naito M, Fujita N, Noguchi K, Tsuruo T(1995) Identification of actin as a substrate of ICE and an ICE-like protease and involvement of an ICE-like protease but not ICE in VP-16-induced U937 apoptosis. Biochem Biophys Res Commun 217: 1185-1192.
Mashima T, Naito M, Noguchi K, Miller D, Nicolson D, Tsuruo T (1997) Actin cleavage by CPP-32/apopain during the development of apoptosis. Oncogene 14: 1007-1012.
McGahon AJ, Martin SJ, Bissonette RP, Mahboubi A, Shi Y, Mogil RJ, Nishioka WK, Green DR (1995) The end of the (cell) line: methods for the study of apoptosis in vitro. Method Cell Biol 46: 153-185.
Medema JP, Scaffidi C, Kirschkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME (1997) FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J 16: 2794-2804.
Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J (1993) Induction of apoptosis in fibroblasts by IL-1?-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 75: 653-660.
Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM (1996) FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/Apo-1) deathinducing signal complex. Cell 85: 817-827.
Nagata S, Goldstein P (1995) The Fas death factor. Science 267: 1449-1456.
Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, Munday NA, Raju SM, Smulson ME, Yamin TT, Yu VL, Miller DK (1995) Identification and inhibition of the ICE-CED-3 protease necessary for mammalian apoptosis. Nature 376: 37-43.
Ohtsu M, Sakai N, Fujita H, Kashiwagi M, Gasa S, Shimizu S, Eguchi Y, Tsujimoto Y, Sakiyama Y, Kobayashi K, Kuzumaki N (1997) Inhibition of apoptosis by the actin-regulatory protein gelsolin. EMBO J 16: 4650-4656.
Owen-Schaub LB, Meterissian S, Ford RJ (1993) Fas/APO-1 expression and function of malignant cells of hematologic and nonhematologic origin. J Immunother 14: 234-241.
Owen-Schaub LB, Radinsky R, Kruzel E, Berry K, Yonehara S (1994) Anti-Fas on nonhematopoietic tumors: levels on Fas/APO-1 and bcl-2 are not predictive of biological responsiveness. Cancer Res 54: 1580-1586.
Pathan NI, Geahlen RL, Harrison ML (1996) The protein-tyrosine kinase lck associates with and is phosphorylated by Cdc2. J Biol Chem 271: 27517-27523.
Piacentini M, Fesus L, Farrace MG, Ghibelli L, Piredda L, Melino G (1991) The expression of ‘tissue’ transglutaminase in two human cancer cell lines is related with the programmed cell death (apoptosis). Eur J Cell Biol 54: 246-254.
Sakahira H, Enari M, Nagata S (1998) Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 391: 96-99.
Samejima K, Tone S, Kottke TJ, Enari M, Sakahira H, Cooke C, Durrieu F, Martins LM, Nagata S, Kauffmann S, Earnshaw WC (1998) Transition from caspase-dependent to caspase-independent mechanism at the onset of apoptotic execution. J Cell Biol 143: 225-239.
Sato T, Irie S, Kitada S, Reed JC (1995) FAP-1: a protein tyrosine phosphatase that associates with Fas. Science 268: 411-415.
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17: 1675-1687.
Shi L, Chen G, He D, Bosc DG, Litchfield DW, Greenberg AH (1996) Granzyme b induced apoptosis and cyclin A-associated cyclindependent kinase activity in all stages of the cell cycle. J Immunol 157: 2381-2385.
Stanger BZ, Leder P, Lee TH, Kim E, Seed B (1995) RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 81: 513-523.
Tewari M, QuanL T, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM (1995) Yama/CPP32β, a mammalian homolog of CED-3, is a crmA-inhibitable protease that cleaves the death substrate poly (ADP-ribose) polymerase. Cell 81: 801-809.
Thornberry NA, Bull HB, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, Elliston KO, Ayala JM, Csano FJ, Chin J, Ding GJF, Egger LA, Gaffney EP, Limjuco G, Palynha OC, Raju SM, Rolando AM, Salley JP, Yamin TT, Lee TD, Shively JE, MacCross M, Mumford RA, Schmidt JA, Tocci MJ (1992) A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature 356: 768-774
Urbani L, Sherwood SW, Schimke R (1995) Dissociation of nuclear and cytoplasmic cell cycle progression by drugs employed in cell synchronization. Exp Cell Res 219: 159-168.
Vincenz C, Dixit VM (1997) Fas-associated death domain protein interleukin-1β-converting enzyme 2 (FLICE2), an ICE/Ced-3 homologue, is proximally involved in CD95-and p55-mediated death signaling. J Biol Chem 272: 6578-6583.
Yanagisawa J, Takahashi M, Kanki H, Yano-Yanagisawa H, Tazunoki T, Sawa E, Nishitoba T, Kamishohara M, Kobayashi E, Kataoka S, Sato T (1997) The molecular interaction of Fas and FAP-1. J Biol Chem 272: 8539-8545.
Zhou BB, Li H, Yuan J, Kirschner MW (1998) Caspase-dependent activation of cyclin-dependent kinases during Fas-induced apoptosis in Jurkat T cells. Proc Natl Acad Sci USA 95: 6785-6790.
Zhang J, Cado D, Chen A, Kabra NH, Winoto A (1998) Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort-1. Nature 392: 296-303.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maruyama, W., Irie, S. & Sato, TA. Morphological Changes in the Nucleus and Actin Cytoskeleton in the Process of Fas-induced Apoptosis in Jurkat T Cells. Histochem J 32, 495–503 (2000). https://doi.org/10.1023/A:1004104619154
Issue Date:
DOI: https://doi.org/10.1023/A:1004104619154