Abstract
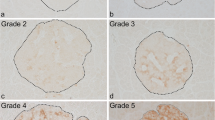
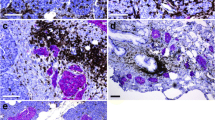
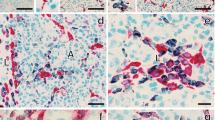
Cyclophosphamide has been used to accelerate and synchronize diabetes in non-obese diabetic (NOD) mice. It was injected to 70-day-old female NOD mice and its effect on the progression of insulitis studied at days 0, 4, 7, 11 and at onset of diabetes. Pancreatic sections were also examined for the influx of CD4 and CD8 T cells and macrophages following immunofluorescence staining. The kinetics of macrophage immunoreactive cells in the exocrine and intra-islet areas were also investigated. Light and confocal microscopy were employed to examine the expression and co-localization of inducible nitric oxide synthase following dual- and triple-label immunofluorescence histochemistry. After cyclophosphamide administration, the severity of insulitis remained similar from days 0 to 4 but began to rise at day 7 and markedly by day 11 and at onset of diabetes. At these two later stages, the insulitis scores were close to 100% while in age-matched control groups the insulitis scores were considerably lower. Immunohistochemical staining showed increasing numbers of CD4 and CD8 T cell subsets and macrophages within the islets and in exocrine, sinusoidal and peri-vascular regions. At onset of diabetes, several islets contained prominent clusters of macrophage immunoreactive cells. Macrophage influx into the islets increased sharply from day 7 (mean number per islet: 119±54 SEM), peaked at day 11 (mean number per islet: 228±42), and then declined at onset of diabetes (mean number per islet: 148±49). Several cells with immunolabelling for inducible nitric oxide synthase were detectable from day 7 onwards until the onset of diabetes. Dual- and triple-label immunohistochemistry showed that a significant proportion of macrophages and only a few beta cells contained the enzyme. Macrophages positive for the enzyme were located as clusters or occasionally contiguously, in the peri-islet and intra-islet areas but rarely in the exocrine region. Islets with minimal distribution of macrophages in the peri-islet areas were not positive for inducible nitric oxide synthase. Beta cells positive for the enzyme were observed in islets with significant macrophage infiltration in locations close to macrophages. The present results show that cyclophosphamide administration to female NOD mice results in a rapid influx of CD4 and CD8 cells and macrophages. The marked up-regulation of inducible nitric oxide synthase in a selective proportion of macrophages, within the islets, immediately preceding and during the onset of diabetes suggests that nitric oxide released by islet macrophages may be an important molecular mediator of beta cell destruction in this accelerated model of insulin-dependent diabetes mellitus.
Similar content being viewed by others
References cited
Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA (1998) IL-1 produced and released endogenously with human islets inhibits β cell function. J Clin Invest 102: 516-526.
Augustein P, Elefanty AG, Allison J, Harrison LC (1998) Apoptosis and beta cell destruction in pancreatic islets ofNODmice with spontaneous and cyclophosphamide-accelerated diabetes. Diabetologia 41: 131-138.
Bacelj A, Charlton B, Mandel TE (1989) Prevention of cyclophosphamideinduced diabetes by anti-Vβ8 T-lymphocyte-receptor monoclonal antibody therapy in NOD/Wehi mice. Diabetes 38: 1492-1495.
Cailleau C, Diu-Hercend A, Ruuth E, Westwood R, Carnaud C (1997) Treatment with neutralizing antibodies specific for IL-1β prevents cyclophosphamide-induced diabetes in nonobese diabetic mice. Diabetes 46: 937-940.
Charlton B, Bacelj A, Mandel TE (1988) Administration of silica particles or anti-Lyt2 antibody prevents β-cell destruction in NOD mice given cyclophosphamide. Diabetes 37: 930-935.
Charlton B, Bacelj A, Slattery RM, Mandel TE (1989) Cyclophosphamide-induced diabetes in NOD/Wehi mice: evidence for suppression in spontaneous autoimmune diabetes mellitus. Diabetes 38: 441-447.
Corbett JA, Wang JL, Sweetland MA, Lancaster JR, McDaniel ML (1992) Interleukin 1β induces the formation of nitric oxide by β-cells purified from rodent islets of Langerhans. Evidence for the β cell as a source and site of action of nitric oxide. J Clin Invest 90: 2384-2391.
Corbett JA, McDaniel ML (1995) Intra-islet release of interleukin 1 inhibits β cell function by inducing β cell expression of inducible nitric oxide synthase. J Exp Med 181: 559-568.
Eisenbarth GS, Connelly J, Soeldner JS (1987) The natural history of type 1 diabetes. Diabetes Metab Rev 3: 873-891.
Eizirik DL, Pavlovic D (1997) Is there a role for nitric oxide in β-cell dysfunction and damage in IDDM? Diabetes Metab Rev 13: 293-307.
Eizirik DL, Pipeleers DG, Ling Z, Welsh N, Hellerstrom C, Andersson A (1994a) Major species differences between humans and rodents in the susceptibility to pancreatic β-cell injury. Proc Natl Acad Sci USA 91: 9253-9256.
Eizirik DL, Sandler S, Welsh N, Cetkovic-cvrlje M, Nieman A, Geller DA, Pipeleers DG, Bendtzen K, Hellerstrom C (1994b) Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest 93: 1968-1974.
Foulis AK, Farquharson MA, Hardman R (1987) Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin-containing islets in Type 1 (insulin-dependent) diabetes mellitus. Diabetologia 30: 333-343.
Fujita T, Yui R, Kusumoto Y, Serizawa Y, Makino S, Tochino Y (1982) Lymphocytic insulitis in a ‘non-obese diabetic (NOD)’ strain of mice: an immunohistochemical and electron microscope investigation. Biomed Res 3: 429-443.
Gepts W (1965) Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14: 619-633.
Harada M (1987) Immune disturbance and pathogenesis of non-obese diabetes-prone (NOD) mice. Exp Clin Endocrinol 89: 251-258.
Harada M, Makino S (1984) Promotion of spontaneous diabetes in nonobese diabetes-prone mice by cyclophosphamide. Diabetologia 27: 604-606.
Horio F, Fukuda M, Hattori M (1990) Peritoneal macrophage can transfer diabetes in NOD mice. Diabetes 39: (Suppl 1), 394.
Jansen A, Cook T, Taylor GM, Largen P, Riveros-Moreno V, Moncada S, Cattell V (1994a) Induction of nitric oxide synthase in rat immune complex glomerulonephritis. Kidney Int 45: 1215-1219.
Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA (1994b) Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and β-cell destruction in NOD mice. Diabetes 43: 667-675.
Kallman B, Burkart V, Kroncke K-D, Kolb-Bachoven V, Kolb H (1992) Toxicity of chemically generated nitric oxide towards pancreatic islet cells can be prevented by nicotinamide. Life Sci 51: 671-678.
Kaneto H, Fujii J, Seo HG, Suzuki K, Matsuoko T-A, Nakamura M, Tatsumi H, Yamasaki Y, Kamada T, Taniguchi N (1995) Apoptotic cell death triggered by nitric oxide in pancreatic β-cells. Diabetes 44: 733-738.
Kasuga A, Maruyama T, Takei I, Shimada A, Kasatani T, Watanabe K, Saruta T, Nakaki T, Habu S, Miyazaki J (1993) The role of cytotoxic macrophages in non-obese diabetic mice: cytotoxicity against murine mastocytoma and beta-cell lines. Diabetologia 36: 1252-1257.
Kasuga A, Nakaki T, Takei I, Takayama S, Ozawa Y, Maruyama T, Miyazaki J, Saruta T (1995) Nitric oxide is important for mouse betacell line killing by peritoneal exudate cells obtained from cyclophosphamide treated non-obese diabetic mice. Endocrine J 42: 259-263.
Kay TWH, Campbell IL, Oxbrow L, Harrison LC (1991a) Overexpression of class I major histocompatibility complex accompanies insulitis in the non-obese diabetic mouse and is prevented by anti-interferon-γ antibody. Diabetologia 34: 779-785.
Kay TWH, Campbell IL, Harrison LC (1991b) Characterization of pancreatic T lymphocytes associated with beta cell destruction in the nonobese diabetic (NOD) mouse. J Autoimmun 4: 263-276.
Kikutani H, Makino S (1992) The murine autoimmune diabetes model: NOD and related strains. Adv Immunol 51: 285-322.
Kleemann R, Rothe H, Kolb-Bachofen V, Xie Q-W, Nathan C, Martin S, Kolb H (1993) Transcription and translation of inducible nitric oxide synthase in the pancreas of prediabetic BB rats. FEBS Lett 328: 9-12.
Kolb-Bachofen V, Epstein S, Kiesel U, Kolb H (1988) Low dose streptozotocin-induced diabetes in mice. Electron microscopy reveals single-cell insulitis before diabetes onset. Diabetes 37: 21-27.
Kroncke K, Steiner L, Fehsel K, Kolb-Bachoven V (1995) Cytokineactivated capillary-forming islet endothelial cells lyse beta-cells via nitric oxide generation. Autoimmunity 21: 42.
Kwon G, Corbett JA, Rodi CP, Sullivan P, McDaniel ML (1995) Interleukin-1β-induced nitric oxide synthase expression by rat pancreatic β-cells: evidence for the involvement of nuclear factor κB in the signalling mechanism. Endocrinology 136: 4790-4795.
Lampeter EF, Signore A, Gale EAM, Pozzilli P (1989) Lessons from the NODmouse for the pathogenesis and immunotherapy of human Type 1 (insulin-dependent) diabetes mellitus. Diabetologia 32: 703-708.
Lee KU, Amano K, Yoon JW (1988) Evidence for initial involvement of macrophage in development of insulitis in NOD mice. Diabetes 37: 989-991.
Lee M-S, Kim, Y-H, Kim K-W (1998) Apoptosis of pancreatic β-cells and lymphocytes detected in accelerated diabetes of NOD mice. Third Immunology of Diabetes Society Conference, Oak Brook, IL, USA, Abstract 40.
Mandrup-Poulsen T, Helqvist S, Molvig J, Wogensen LD, Nerup J (1989) Cytokines as immune effector molecules in autoimune endocrine diseases with special reference to insulin-dependent diabetes mellitus. Autoimmunity 4: 191-218.
Mandrup-Poulsen T, Helqvist S, Wogensen LD, Molvig J, Pociot F, Johannesen J, Nerup J (1990) Cytokines and free radicals as effector molecules in the destruction of pancreatic beta cells. In: Baekkeskov S, Hansen B eds. Human Diabetes — Genetic, Environmental and Autoimmune Etiology. Heidelberg: Springer-Verlag, pp. 169-193.
Qin HY, Singh B (1997) BCG vaccination prevents insulin-dependent diabetes mellitus (IDDM) in NOD mice after disease acceleration with cyclophosphamide. J Autoimmun 10: 271-278.
Rabinovitch A (1993) Roles of cytokines in IDDM pathogenesis and islet β-cell destruction. Diabetes Rev 1: 215-240.
Rabinovitch A, Sumoski W, Rajotte RV, Warnock GL (1990) Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab 71: 152-156.
Rabinovitch A, Suarez-Pinzon WL, Srydnadka K, Lakey JRT, Warnock GL, Rajotte RV (1994) Human pancreatic islet β-cell destruction by cytokines is independent of nitric oxide production. J Clin Endocrinol Metab 79: 1058-1062.
Rabinovitch A, Suarez-Pinzon WL, Sorensen O, Bleackley RC (1996) Inducible nitric oxide synthase (iNOS) in pancreatic islets of nonobese diabetic mice: identification of iNOS expressing cells and relationships to cytokines expressed in the islets. Endocrinology 137: 2093-2099.
Reddy S, Bibby NJ, Elliott RB (1988) Ontogeny of islet cell antibodies, insulin autoantibodies and insulitis in the non-obese diabetic mouse. Diabetologia 31: 322-328.
Reddy S, Bibby NJ, Elliott RB (1991) Time course of immune markers in the NOD mouse: comparison between sexes and animals on an antidiabetogenic diet. Diabetes Nutrition Metab Clin Exp 4: 259-265.
Reddy S, Liu W, Elliott RB (1993) Distribution of pancreatic macrophages preceding and during insulitis in young NOD mice. Pancreas 8: 602-608.
Reddy S, Wu D, Swinney C, Elliott RB (1995) Immunohistochemical analyses of pancreatic macrophages and CD4 and CD8 T cell subsets prior to and following diabetes in theNODmouse. Pancreas 11: 16-25.
Reddy S, Kaill S, Poole CA, Ross J (1997) Inducible nitric oxide synthase in pancreatic islets of the non-obese diabetic mouse: a light and confocal microscopical study of its ontogeny, co-localization and up-regulation following cytokine administration. Histochem J 29: 53-64.
Rothe H, Faust A, Schade U, Kleemann R, Bosse G, Hibino T, Martin S, Kolb H (1994) Cyclophosphamide treatment of female non-obese diabetic mice causes enhanced expression of inducible nitric oxide synthase and interferon-gamma, but not of interleukin-4. Diabetologia 37: 1154-1158.
Rothe H, O'Hara RM, Martin S, Kolb H (1997) Suppression of cyclophosphamide induced diabetes development and pancreatic Th1 reactivity in NOD mice treated with the interleukin (IL)-12 antagonist IL-12(p40)2. Diabetologia 40: 641-646.
Sai P, Rivereau AS, Granier C, Haertle T, Martignat L (1996) Immunization of non-obese diabetic (NOD) mice with glutamic acid decarboxylase-derived peptide 524-543 reduces cyclophosphamideaccelerated diabetes. Clin Exp Immunol 105: 330-337.
Sandler S, Andersson A, Hellerstrom C (1987) Inhibitory effects of interleukin-1 on insulin secretion, insulin biosynthesis, and oxidative metabolism of isolated rat pancreatic islets. Endocrinology 121: 1424-1431.
Sandler S, Eizirik DL, Svensson C, Strandell E, Welsh M, Welsh N (1991) Biochemical and molecular actions of interleukin-1 on pancreatic β-cells. Autoimmunity 10: 241-253.
Shimada A, Takei I, Maruyama T et al. (1992) Transfer of peritoneal macrophages in NOD mice. Diabetes 41 (Suppl 1), 506, Abstract.
Signore A, Pozzilli P, Gale EAM, Andreani D, Beverley PCL (1989) The natural history of lymphocyte subsets infiltrating the pancreas of NOD mice. Diabetologia 32: 282-289.
Southern C, Schulster D, Green IC (1990) Inhibition of insulin secretion by interleukin-1β and tumour necrosis factor-β by an L-argininedependent nitric oxide generating mechanism. FEBS Lett 276: 42-44.
Sumoski W, Baquerizo H, Rabinovitch A (1989) Oxygen free radical scavengers protect rat islet cells from damage by cytokines. Diabetologia 32: 792-796.
Taki T, Nagata M, Ogawa W, Hatamori N, Hayakawa M, Hari J, Shii K, Baba S, Yokono K (1991) Prevention of cyclophosphamide-induced and spontaneous diabetes in NOD/Shi/Kbe mice by anti-MHC class I Kd monoclonal antibody. Diabetes 40: 1203-1209.
Welsh N, Eizirik DL, Bendtzen K, Sandler S (1991) Interleukin-1β induced nitric oxide production in isolated rat pancreatic islet requires gene transcription and may lead to inhibition of the Krebs cycle enzyme, aconitase. Endocrinology 129: 3167-3173.
Whittle BJR (1995) Nitric oxide in physiology and pathology. Histochem J 27: 727-737.
Xie Q-W, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Ding A, Troso T, Nathan C (1992) Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256: 225-228.
Yasunami R, Bach J-F (1988) Anti-suppressor effect of cyclophosphamide on the development of spontaneous diabetes in NOD mice. Eur J Immunol 18: 481-484.
Zhang ZL, Georgious HM, Mandel TE (1993) The effect of cyclophosphamide treatment on lymphocyte subsets in the non-obese diabetic mouse: a comparison of various lymphoid organs. Autoimmunity 15: 1-10.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reddy, S., Yip, S., Karanam, M. et al. An Immunohistochemical Study of Macrophage Influx and the Co-localization of Inducible Nitric Oxide Synthase in the Pancreas of Non-obese Diabetic (NOD) Mice During Disease Acceleration with Cyclophosphamide. Histochem J 31, 303–314 (1999). https://doi.org/10.1023/A:1003765918017
Issue Date:
DOI: https://doi.org/10.1023/A:1003765918017