Abstract
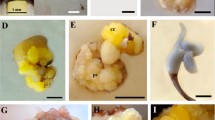
An efficient protocol for plant regeneration from protoplasts of peppermint ‘Mitcham Digne 38’, ‘Mitcham Ribecourt 19’ and ‘Todd's#x2019; was developed by stepwise optimization of first cell division, microcalli formation and shoot differentiation. The rate of first cell divisions was strongly dependent on the addition of 2,4-D to callus induction medium. Best results were obtained with 1 μM 2,4-D in combination with NAA (2.5 μM) and BA (4 μM). Although liquid medium was more efficient to support first protoplast divisions, solid medium was clearly more suitable to sustain subsequent cell divisions leading to the formation of microcalli. Shoot organogenesis was induced from protoplast-derived calli by using reduced auxin concentration (0.5 μM NAA) and high concentration of cytokinins. Addition of 2.3 μM thidiazuron increased bud formation, allowing a regeneration frequency of more than 50% from calli of ‘Mitcham Digne 38’ and ‘Todd's’. Genotypic differences were noticed for regeneration capability and the pathway of shoot regeneration.
Similar content being viewed by others
References
Banthorpe DV (1996) Mentha species (mints): in vitro culture and production of lower terpenoids and pigments. In: Bajaj PS (eds) Biotechnology in Agriculture and Forestry, Vol. 37 Medicinal and Aromatic Plants IX (pp 202–225) Springer-Verlag, Berlin
Böhmer P, Meyer B & Jacobsen H-J (1995) Thidiazuron-induced high frequency of shoot induction and plant regeneration in protoplast derived pea callus. Plant Cell Rep. 15: 26–29
Brown SC, Bergounioux C, Tallet S & Marie D (1991) Flow cytometry of nuclei for ploidy and cell cycle analysis. In: Negrutiu I & Gharti-Chhetri G (eds) A Laboratory Guide for Cellular and Molecular Plant Biology, Birkhäuser Verlag, Basel
Caissard JC, Faure O, Jullien F, Colson M & Perrin A (1996) Direct regeneration in vitro and transient GUS expression in Mentha × piperita. Plant Cell Rep. 16: 67–70
Chaput MH, San H, De Hys L, Grenier E, David H & David A (1996) How plant regeneration from Mentha × piperita L. and Mentha × citrata Ehrh. leaf protoplasts affects their monoterpene composition in field conditions. J. Plant Physiol. 149: 481–488
Frearson EM, Power JB & Cocking EC (1973) The isolation, culture and regeneration of Petunia leaf protoplasts. Dev. Biol. 33: 130–137
Gamborg OL, Miller RA & Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158
Morel G & Wetmore RH (1951) Tissue culture of monocotyledons. Am. J. Bot. 38: 138–140
Panis B, Van Wauwe A & Swennen R (1993) Plant regeneration through direct somatic embryogenesis from protoplasts of banana (Musa spp.) Plant Cell Rep. 12: 403–407
Rech EL & Pires MJP (1986) Tissue culture propagation of Mentha sp. by the use of axillary buds. Plant Cell Rep. 5: 17–18
Sato H, Enomoto S, Oka S, Hosomi K & Ito Y (1993) Plant regeneration from protoplasts of peppermint (Mentha x piperita L.). Plant Cell Rep. 12: 546–550
Sato H, Enomoto S, Oka S, Hosomi K & Ito Y (1994) The effect of 4-PU on protoplast culture of peppermint (Mentha piperita L.). Plant Tissue Cult. Lett. 11: 134–138
Sato H, Yamada K, Mii M, Hosomi K, Okuyma S, Uzawa M, Ishikawa H & Ito Y (1996) Production of an interspecific somatic hybrid between peppermint and gingermint. Plant Sci. 115: 101–107
Tegeder M, Gebhardt D, Schieder O & Pickardt T (1995) Thidiazuron-induced plant regeneration from protoplasts of Vicia faba cv. Mythos. Plant Cell Rep. 15: 164–169
Thomas JC & Katterman FR (1986) Cytokinin activity induced by thidiazuron. Plant Physiol. 81: 681–683
Van Eck JM & Kitto SL (1990) Callus initiation and regeneration in Mentha. HortScience 25: 804–806
Wang D, Miller PD & Söndahl MR (1989) Plant regeneration from protoplasts of Indica type rice and CMS rice. Plant Cell Rep. 8: 329–332
Wei ZM & Xu ZH (1990) Plant regeneration from protoplasts of immature cotyledons of Glycine soja Sieb. et Zucc. Plant Sci. 70: 101–104
Widholm JM (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 47: 189–194
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jullien, F., Diemer, F., Colson, M. et al. An optimising protocol for protoplast regeneration of three peppermint cultivars ( Mentha x piperita). Plant Cell, Tissue and Organ Culture 54, 153–159 (1998). https://doi.org/10.1023/A:1006185103897
Issue Date:
DOI: https://doi.org/10.1023/A:1006185103897