Middle East respiratory syndrome coronavirus (MERS-CoV) is a novel β-coronavirus identified in 2012.Reference Zaki, van Boheemen, Bestebroer, Osterhaus and Fouchier 1 Infection may result in upper or lower respiratory tract illness, with symptoms ranging from inapparent or mild to rapidly progressive respiratory failure and, in ~35% of confirmed cases, death.Reference Arabi, Balkhy and Hayden 2 Numerous large, healthcare-associated outbreaks of MERS-CoV have occurred, resulting in transmission to patients, visitors, and healthcare personnel (HCP).Reference Assiri, McGeer and Perl 3 – Reference Assiri, Abedi and Bin Saeed 6
Prevention of MERS-CoV transmission in healthcare settings requires effective triaging and a high clinical index of suspicion to facilitate early recognition of suspect cases, followed by implementation of appropriate infection prevention and control (IPC) measures.Reference Balkhy, Alenazi and Alshamrani 4 MERS-CoV may be transmitted by close contact that likely includes respiratory droplets, but transmission routes are still not fully understood.Reference Bin, Heo and Song 7 Both the Saudi Arabia Ministry of Health (MoH) and the World Health Organization (WHO) recommend MERS-CoV-specific precautions for healthcare settings to reduce the risk of healthcare-associated transmission.Reference Al-Abdely, Kasole and AlRajhi 8 , 9
From May 28 through June 19, 2017, 2 hospitals in Riyadh, Saudi Arabia, reported MERS CoV outbreaks; epidemiologic links between the 2 hospitals were not apparent, and the extent of circulation was unknown. The outbreaks were initially reported by WHO in July 2017. 10 The MoH and US Centers for Disease Control and Prevention (CDC) conducted an investigation to describe the scope of healthcare-associated transmission using epidemiologic, molecular, and serologic methods.
Methods
Setting
The investigation was conducted at 4 healthcare facilities in Riyadh, Saudi Arabia, where cases presented: (1) hospital A, a 1,200-bed tertiary-care MoH hospital with 140 intensive-care unit (ICU) beds and a busy emergency department (ED); (2) hospital B, a 200-bed MoH specialty pulmonary hospital; (3) clinic C, an outpatient clinic; and (4) an outpatient dialysis unit.
Outbreak investigation
We defined a case as any patient with laboratory-confirmed MERS-CoV infection and an epidemiologic connection to the affected healthcare facilities as a patient, HCP, visitor or family member of a patient from May 28 through June 19, 2017. Laboratory confirmation was performed either at the MoH using a rRT-PCR assay targeting both the region upstream of the E gene (UpE) and open reading frame (ORF) 1a 11 , Reference Corman, Muller and Costabel 12 or at the CDC by genome sequencing. An indeterminate rRT-PCR result was defined as positive result on only 1 of the 2 gene targets required for confirmation. We defined a superspreading event as any interaction in which a MERS case transmitted to ≥5 subsequent cases.
Patients with symptoms consistent with MERS and contacts exposed to identified cases were tested. Contact investigations were performed by hospital infection control personnel, local public health authorities, and MoH personnel. MoH recommends MERS-CoV testing of HCP identified with prolonged, close contact with a MERS case (ie, >10 minutes within 1.5 m) if not properly wearing personal protective equipment (PPE). 13 In addition to recommended testing, ad hoc testing of HCP contacts with various levels of exposure and PPE use occurred. We reviewed available medical and public health records for all cases and conducted key-informant interviews with HCP.
We collected sera and interviewed available HCP cases and HCP identified as rRT-PCR–negative contacts. Interview forms included questions related to demographics, occupation, exposures, PPE use, symptoms, and underlying medical conditions.
Methods
Genome sequencing and phylogenetic analysis
Available MERS-CoV rRT-PCR–positive samples from confirmed cases collected from May 28 through June 19, 2017, were stored at −80°C and shipped to the CDC for further molecular analysis. Sample aliquots (200 µL) were extracted on a NucliSens EasyMAG (BioMerieux, Marcy-l'Étoile, France), and 100 µL of total nucleic acid was recovered. The specimen extracts were retested by MERS-CoV N2 and/or N3 real-time rRT-PCR assays,Reference Lu, Whitaker and Sakthivel 14 and genome sequencing was performed on positive samples with sufficient viral load using the previously described primer sets and protocol.Reference Assiri, Midgley and Abedi 15 , Reference Yusof, Queen and Eltahir 16
The nucleotide sequences were first aligned in MAFFT version 7.013 multiple-sequence alignment software. Phylogenetic trees were inferred using the maximum likelihood (ML) method with PhyML version 3.0 software,Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel 17 assuming a general time-reversible (GTR) model with a discrete γ-distributed rate variation among sites (γ4) and an SPR tree-swapping algorithm and visualized using MEGA version 6 software.Reference Tamura, Stecher, Peterson, Filipski and Kumar 18
Serology
Serum samples were tested at the CDC for anti-MERS-CoV antibodies using indirect ELISAs for nucleocapsid (N) and spike (S) proteins followed by a confirmatory microneutralization test (MNT) as previously described.Reference Trivedi, Miao and Al-Abdallat 19 At the optical density cutoffs used by our laboratory, the N ELISA has a sensitivity of 88.9% and a specificity of 92.2%, and the S ELISA has a sensitivity of 90.8% and a specificity of 90.8% (unpublished data). MERS-CoV seropositivity was defined as having 2 of 3 positive assays, including N-ELISA, S-ELISA, and MNT, or positive by MNT alone. Indeterminate seropositivity was defined as S ELISA positive, but N ELISA and MNT-negative.
Ethics
This investigation was determined by MoH and CDC to be public health response, not research, and therefore was not subject to institutional review board (IRB) review. Signed consent was obtained from seroepidemiologic investigation participants. Interviews were conducted in Arabic, English, Filipino, or Malayalam.
Results
Outbreak investigation
We identified 48 MERS cases, including 38 linked to hospital A and 10 linked to hospital B (Fig. 1 and Table 1). At both hospitals, transmission was traced to a single introduction by the respective index cases (Fig. 2). Index patient A presented at hospital A on May 28, and index patient B presented at hospital B on June 2. No epidemiologic link was established between these cases.
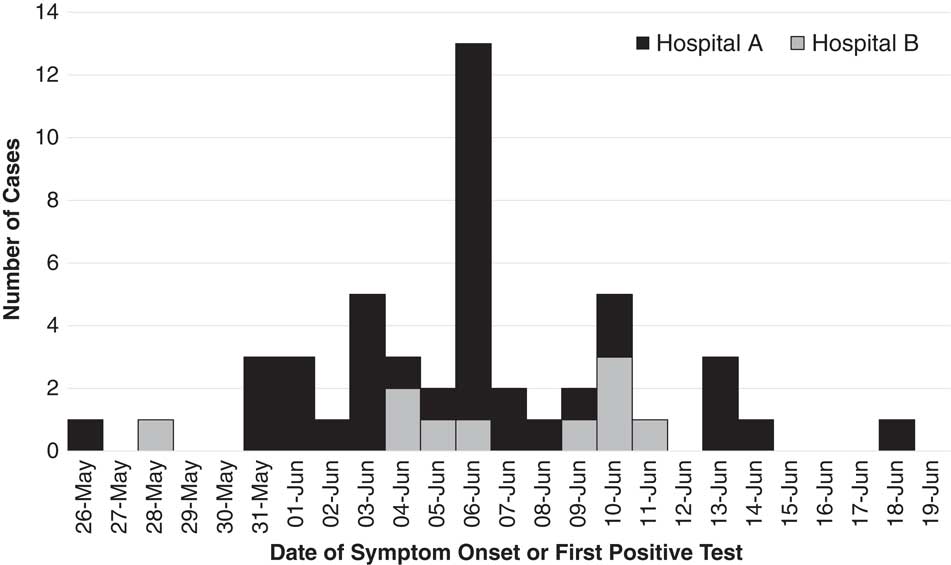
Fig. 1 Middle East respiratory syndrome (MERS) cases associated with hospital A (n=38) and hospital B (n=10) outbreaks, Riyadh, Saudi Arabia, from May 28 through June 19, 2017.
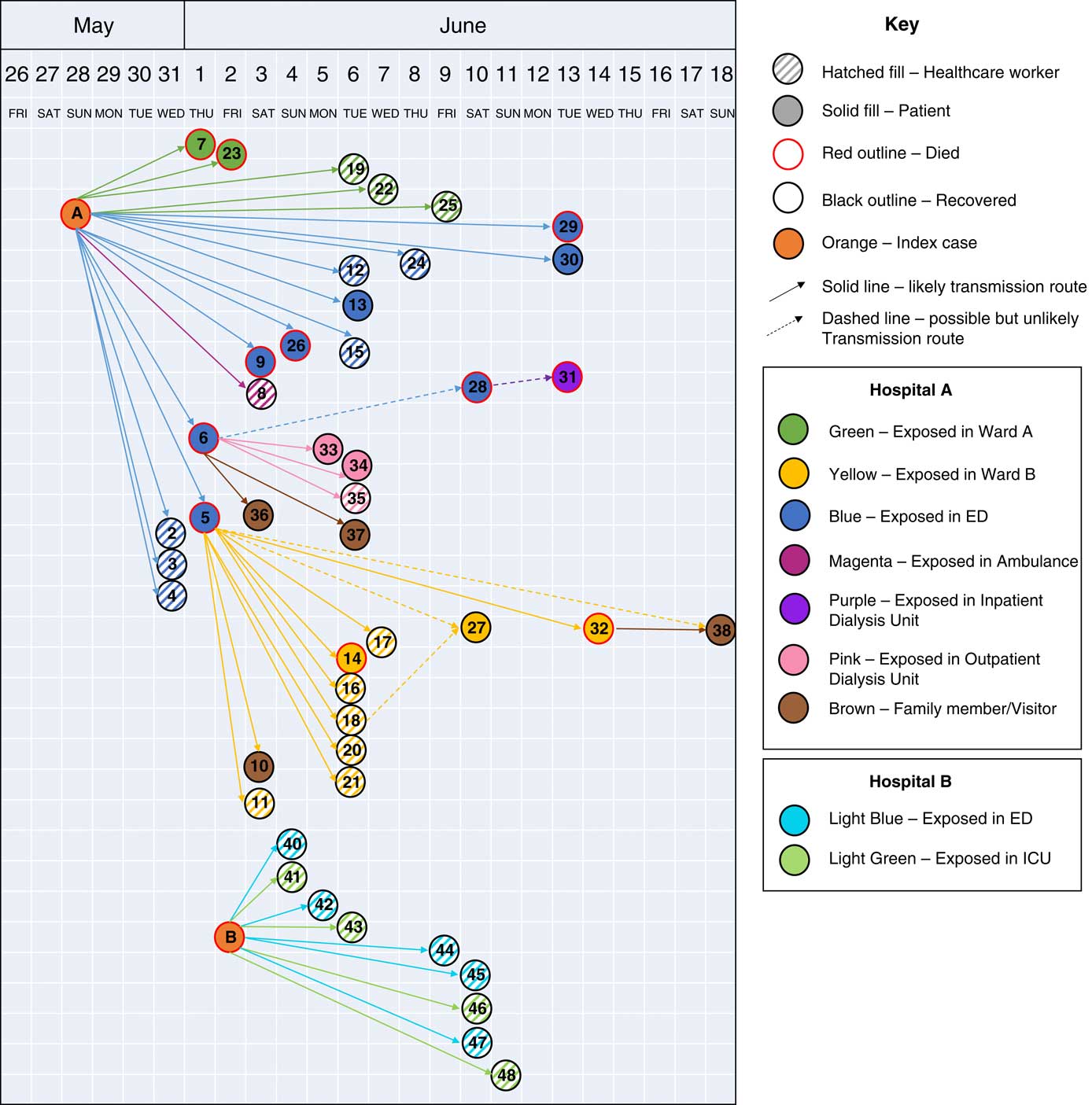
Fig. 2 Transmission of MERS-CoV infections between cases at hospital A, an outpatient dialysis unit, and hospital B, Riyadh, Saudi Arabia, from May 28 through June 19, 2017. Cases are shown by date of symptom onset or positive real-time RT-PCR test, except index cases, which are shown by date of hospitalization.
Table 1 Demographics of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Cases (N=48)
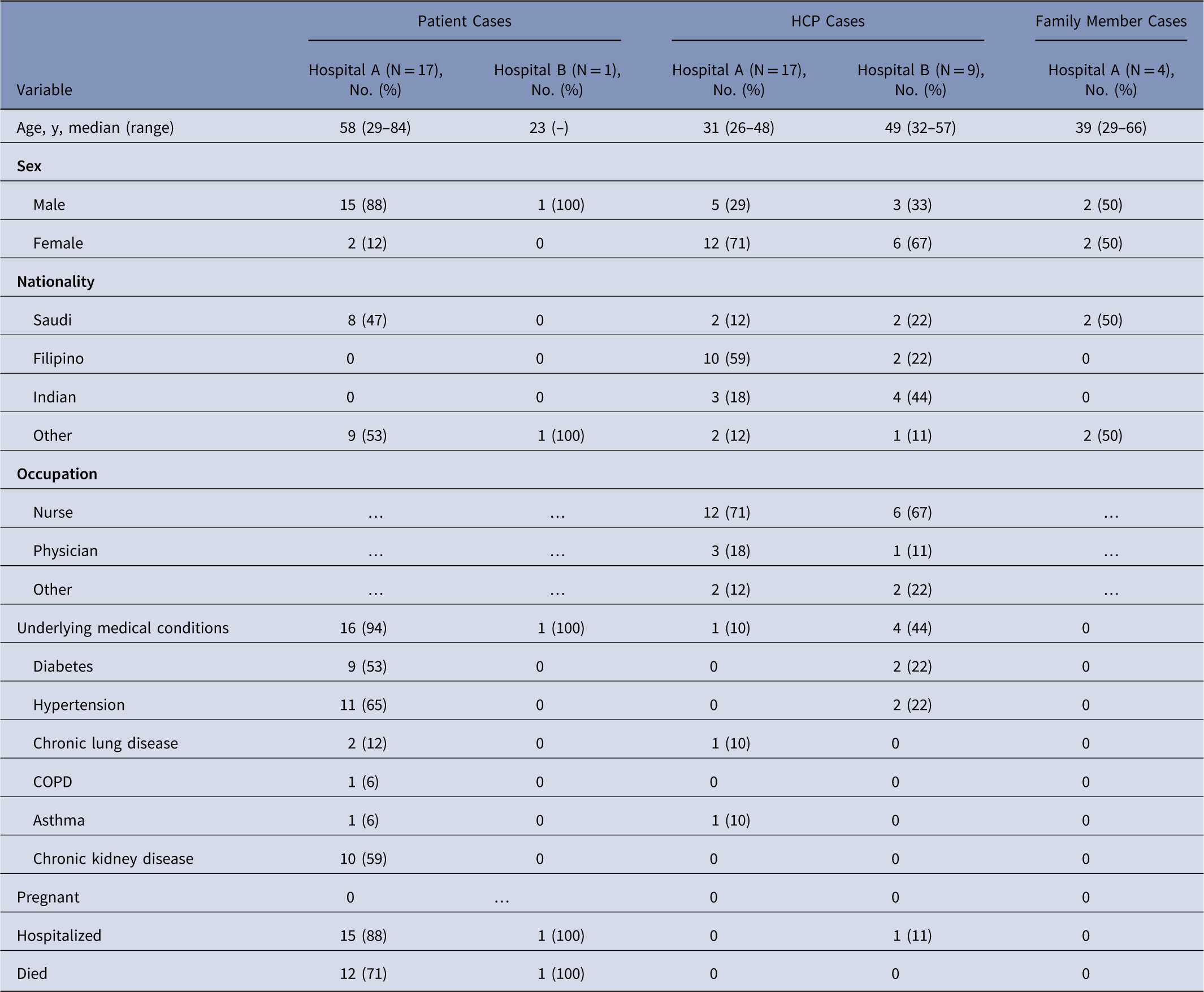
Note. HCP, healthcare personnel; COPD, chronic obstructive pulmonary disease.
Respiratory specimens from 36 MERS-CoV cases were received by the CDC: 35 were confirmed positive by rRT-PCR and 1 positive specimen could not be confirmed by MERS-CoV N2 and/or N3 rRT-PCR assays but was confirmed by sequencing the spike gene. Phylogenetic analysis of 95 MERS-CoV genomes, including 21 complete or nearly complete genomes in this study, showed clustering of the outbreak sequences in lineage 5 within clade BReference Assiri, Midgley and Abedi 15 , Reference Wang, Liu and Shi 20 (Fig. 4). The outbreak sequences from each hospital formed a monophyletic group and separated into 2 distinct clusters, suggesting 2 distinct outbreaks. The hospital A cluster appears to have been closely related to camel MERS-CoV (KT368879) and human MERS-CoV (MG011358) sampled at Riyadh in 2015 and 2016 respectively. The hospital B cluster appears to have been more related to several human MERS-CoV sampled from Riyadh in 2016 (KX154684, MG011362, KX154693) (Fig. 4).
Hospital A
Among 38 cases linked to hospital A, 17 were patient cases, 17 were HCP cases, and 4 were family members (Table 1). Index patient A was a 46-year-old factory worker with no history of contact with camels or camel products. He presented to the ED on May 28 with cough, shortness of breath, and chest pain. Although ER triage was in place, the patient was not initially considered a suspected MERS case, and he remained in the ED for >14 hours prior to transfer to a medical ward (ward A). Index patient A was directly linked to 19 subsequent cases: 1 ambulance driver exposed in the ambulance, 13 likely exposed in the ED, and 5 on ward A, where index A was intubated without airborne precautions in place. On hospital day 3, index patient A was suspected of MERS and was transferred from ward A to a negative-pressure room with recommended isolation precautions. Index patient A was confirmed rRT-PCR positive for MERS-CoV on May 31, and contact tracing began the same day. All secondary transmission at hospital A likely occurred before suspicion of MERS in individual cases and the subsequent implementation of recommended transmission-based precautions.
In addition, 2 secondary cases, cases 5 and 6, overlapped with index patient A in the ED and were themselves associated with subsequent superspreading events (Table 2). Initially, case 6 was not identified as a contact of index patient A and was suspected to have had community exposure. However, later medical record review demonstrated overlap with index patient A during his initial ED visit for non-MERS illness on May 28. He subsequently visited an outpatient dialysis unit, followed by a second ED presentation with admission to hospital A on June 1. Case 6 was directly linked to 6 secondary cases: 1 patient at hospital A, 2 patients and 1 cleaner at the outpatient dialysis unit, and 2 household contacts. Molecular evidence showed that case 6 clustered with index A and other subsequent cases at hospital A.
Table 2 Hospitalization and Demographic Details of Cases Linked to ≥5 Secondary Cases
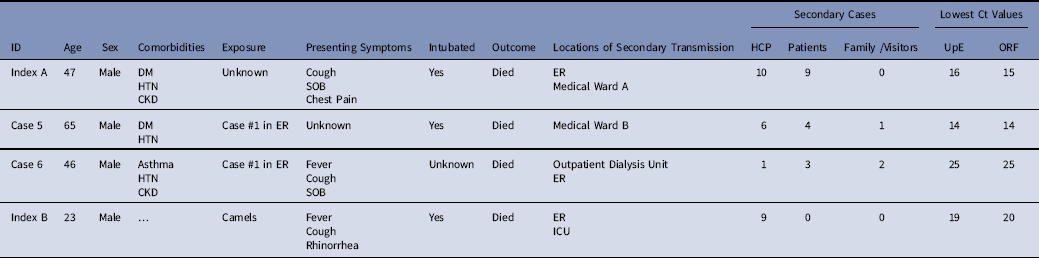
Note. SOB, shortness of breath; DM, diabetes mellitus; CKD, chronic kidney disease; Ct, cycle threshold; HTN, hypertension; ER, emergency room; ICU, intensive care unit; HCP, healthcare personnel; UpE, upstream of the E gene; ORF, open reading frame.
Case 5 was a known contact of index patient A in the ED, where he stayed for 2 days before transfer to a medical ward (ward B). He remained on ward B for 3 days, where he developed respiratory distress and was intubated on June 1. On June 2, MERS was suspected, airborne precautions were implemented, and a sample was obtained for testing. MERS-CoV was confirmed on June 3, and the patient died the same day. Case 5 was linked to 10 subsequent cases on ward B, including 6 HCP (Fig. 2), 4 of whom were present during the intubation procedure on case 5.
Of 17 HCP cases linked to hospital A, 10 were available for interview and serum collection. All 10 interviewed HCP cases reported ≥1 symptom when tested for MERS-CoV, with most HCP cases reporting mild upper-respiratory symptoms and/or diarrhea; none developed severe illness, and all survived. Of these 10 available HCP, 9 reported prolonged, close contact with an unrecognized patient case before implementation of MERS-CoV IPC measures and with limited PPE use (Table 3). The remaining HCP case cared for a non-MERS patient in the same room as a MERS patient case. Of these 10 HCP cases, 4 reported having been in the same room as a patient case during intubation, and none reported wearing an N95 mask or a powered air purifying respirator (PAPR).
Table 3 Exposure to Known MERS Cases and Reported PPE Use Among interviewed HCP Cases who Reported Contact with a Confirmed MERS Case (N=16), Hospitals A&B
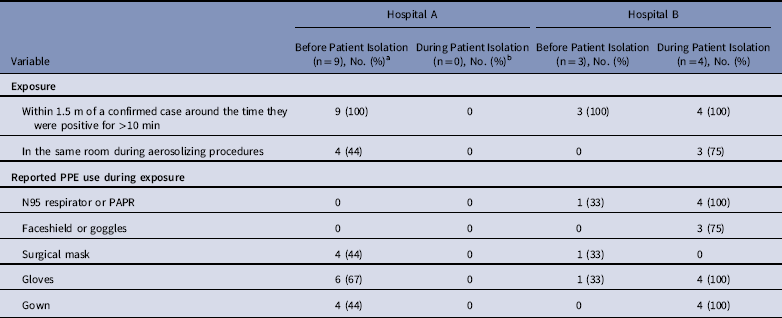
Note. MERS, Middle East respiratory syndrome; PPE, personal protective equipment; HCP, healthcare personnel.
a One HCP denied any contact with a confirmed case when interviewed, reported only contact with a non-MERS case patient on Ward A and was excluded from this table.
b No transmission at hospital A was associated with exposure during isolation.
Among the 10 interviewed HCP cases, the time from first positive MERS-CoV result to serum collection was 55–61 days, and 1 was seropositive: a 32-year-old female who had reported headache, muscle aches, and productive cough. Additionally, we interviewed and collected serum from 66 HCP contacts of cases; none were seropositive.
Among all 15 HCP cases identified at hospital A and the ambulance driver, 8 tested positive on their first rRT-PCR test, and among these 8, the median time from likely exposure to positive sample collection was 5.5 days (range, 3–11 days). The 8 HCP cases who did not test positive on their initial test, tested positive on a second or later test, with a median time from likely exposure to first positive sample collection of 8 days (range, 5–12) (Fig. 3).
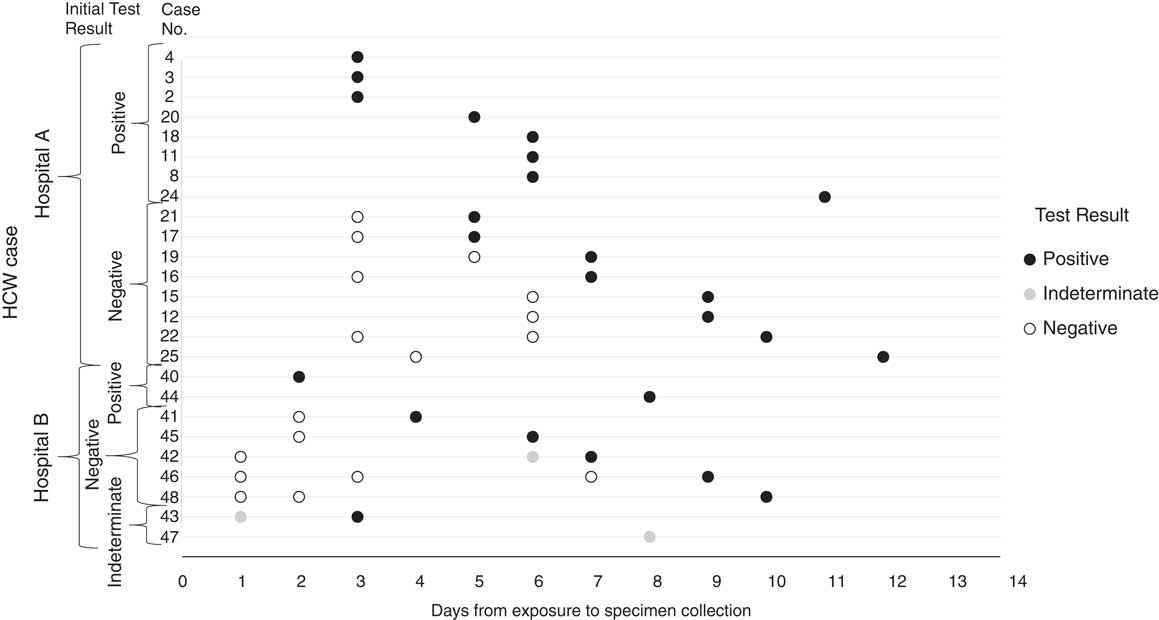
Fig. 3 Real-time reverse-transcription polymerase chain reaction (rRT-PCR) testing results from the date of exposure to the date of first rRT-PCR positive result for healthcare personnel (HCP) cases at hospitals A and B.
Hospital B and clinic C
Ten cases were identified at hospital B; index patient B and 9 HCP cases who reported direct contact with him. Index patient B was a 23-year-old butcher who slaughtered camels and contacted camel products. On May 28, he developed fever, cough, and rhinorrhea and presented to clinic C. He was discharged home but returned to clinic C 3 times over 4 days with worsening respiratory symptoms. On June 1, he was diagnosed with pneumonia and cardiomegaly and was referred to hospital B, where he presented to the ED on June 2. He was not initially suspected to have MERS; however, a chest radiograph revealed bilateral infiltrates and additional history indicated camel contact. He was then placed on isolation precautions, and specimens were collected for MERS-CoV testing. He was intubated later that day after IPC measures for MERS-CoV had been implemented, including transfer to a negative pressure room. He died on June 3.
At clinic C, index patient B had 15 HCP contacts, including 2 with close, prolonged contact; no rRT-PCR confirmed HCP-cases were documented at clinic C. Of 15 HCP contacts of index B, 14 (93%) were interviewed and had serum collected. Among these, 2 HCP were seropositive; both were physicians with initial indeterminate rRT-PCR test results. Subsequent rRT-PCR testing was negative, and neither was recorded as a MERS case. Both cared for index B during multiple clinic visits and reported being within 1.5 m of index patient B for <10 minutes. One reported no PPE use, and the other reported wearing gloves and a surgical mask. Both had diabetes, and 1 reported hypertension and smoking. Both developed symptoms within 4–10 days of caring for index B, including fever.
At hospital B, 27 healthcare contacts of index patient B were identified. Among them, 9 HCP (33%) tested rRT-PCR positive for MERS-CoV; 5 reported contact with index patient B before isolation, and 4 reported contact following implementation of IPC measures for MERS-CoV in a negative-pressure room. Of these latter 4, 3 participated in aerosolizing procedures, including intubation, open suctioning of airways, and/or cardiopulmonary resuscitation. All 3 reported wearing full PPE, including gloves, gown, N95 mask, and face shield. Also, of these 4 HCP, 2 reported visible contamination of gloves or gown by bodily fluids during care of index patient B, who was reported to have had copious respiratory secretions. No transmission to patients or visitors at hospital B was identified.
Of the 9 HCP cases, 7 were interviewed and had serum collected; all 7 reported close, prolonged contact with index patient B. Time from symptom onset to serum collection was 39–47 days. Among these 7 HCP, 3 were seropositive, and 2 had an indeterminate result. Among the 3 seropositive HCPs, 2 had been diagnosed with pneumonia, 1 of whom also had diabetes mellitus. The third reported productive cough, dyspnea, and diabetes mellitus. Among the 2 with an indeterminate result, 1 reported rhinorrhea and nonproductive cough, and the other had fever and upper respiratory tract and gastrointestinal symptoms; neither had comorbidities. The 2 seronegative HCP-cases reported mild upper-respiratory-tract symptoms; 1 also had fever and gastrointestinal symptoms. All 9 survived, and none were critically ill.
At hospital B, 34 of 50 MERS-CoV rRT-PCR–negative HCP contacts of cases (68%) were interviewed and provided serum. One was seropositive, a physician who had close, prolonged contact with index B after isolation and while wearing recommended PPE; however, he had previously tested rRT-PCR positive for MERS-CoV in 2013.
Of 9 HCP cases identified at hospital B, 2 tested positive by rRT-PCR on their first test, 5 tested negative then subsequently tested positive, and 2 had an initial indeterminate rRT-PCR test result (Fig. 3). One HCP case with an initial indeterminate result was subsequently confirmed by rRT-PCR, the other was confirmed by genome sequencing. For the 8 HCP cases with a positive rRT-PCR test, the median time from known exposure to positive sample collection was 6.5 days (range, 2–10 days).
Discussion
A large MERS-CoV transmission event occurred in Riyadh during May–June 2017, with cases initially reported from 2 hospitals. Our molecular and epidemiologic investigation demonstrated separate virus introductions at the 2 facilities, each by a single index case. Similar to previous outbreaks,Reference Assiri, McGeer and Perl 3 , Reference Cowling, Park, Fang, Wu, Leung and Wu 21 , Reference Alenazi, Al Arbash and El-Saed 22 transmission was characterized by early superspreading events, which led to a rapidly escalating number of cases.
During these 2 outbreaks, delays in the recognition and isolation of early cases, along with emergency intubation (sometimes precluding recommended airborne precautions), were associated with superspreading events. Cases linked to superspreading events included 2 index cases and 2 secondarily infected hospitalized cases; all had severe illness, low cycle threshold values suggesting high viral loads, and all 4 died. These results are consistent with prior evidence that length of patient hospitalization before isolation and high viral loads have been linked to transmission.Reference Kim, Park and Jung 23 Although 2 of the cases associated with superspreading events were contacts of index A, they were not detected via contract tracing before developing symptoms and were associated with additional healthcare-associated transmission.
The delay in recognition of index patient A due to the patient’s comorbidities and complex presentation has been previously described.Reference Amer, Alqahtani, Alzoman, Aljerian and Memish 24 This case patient was admitted to the ED without respiratory precautions despite initial triage, highlighting the need for strengthening triage practices. The presentation of index patient B before hospitalization is notable; this 23-year-old male had no known comorbidities and initially presented to clinic C with a mild illness, followed by further visits with worsening respiratory symptoms. Furthermore, 2 physicians at clinic C tested seropositive after an indeterminate rRT-PCR test, suggesting transmission at clinic C. Thus, increased testing for MERS-CoV in an outpatient setting for individuals with known risk factors and worsening respiratory symptoms might facilitate early recognition of MERS cases.
Transmission from patient cases to HCP participating in aerosolizing procedures prior to airborne precautions was likely, despite taking recommended airborne precautions and wearing appropriate PPE. MERS-CoV has been detected in large quantities in respiratory secretionsReference Corman, Albarrak and Omrani 25 and live virus isolated from environmental surfaces.Reference Bin, Heo and Song 7 It is possible that inappropriate use of PPE (eg, insufficient fit testing) or contamination of PPE and inappropriate doffing resulted in transmission. Transmission to HCP wearing isolation gowns and N95 respirators during intubation has been observed previously.Reference Kim, Choi and Jung 26 , Reference Park, Ko and Peck 27 HCP should ensure appropriate fit testing and donning and doffing of PPE to prevent MERS-CoV transmission.
Among the 17 HCP cases tested by serology, 11 (65%) had no detectable antibodies. The 4 seropositive HCP cases (24%) each had either evidence of pneumonia or symptoms suggestive of lower respiratory tract infection, consistent with previous evidence that HCP cases with lower-respiratory-tract symptoms are more likely to have detectable antibodies.Reference Alshukairi, Khalid and Ahmed 28 The use of serologic testing to detect unrecognized infections in asymptomatic or mildly symptomatic individuals may be limited.Reference Payne, Biggs, Al-Abdallat, Alqasrawi, Lu, Abedi, Haddadin, Iblan, Alsanouri, Al Nsour and Sheikh 29
In both outbreaks, rapid identification of contacts, symptom monitoring, and repeated testing allowed for efficient detection of secondary HCP cases and provided information to guide outbreak management. Of the 25 HCP-cases, 10 were detected on initial rRT-PCR testing and 15 by repeated rRT-PCR testing, including multiple HCP cases who initially tested rRT-PCR–negative up to 7 days after known case exposure, indicating that asymptomatic or mildly symptomatic HCP may require repeated screening to rule out infection. Although we found no evidence of transmission from HCP to HCP, rapid furlough of MERS-CoV positive HCP is important to avoid exposing susceptible individuals, particularly patients, to MERS-CoV positive HCP.
Our investigation had several limitations. Complete medical records were not available for all patients. Seropositivity may have been a result of unknown exposures outside of this outbreak. Although hospitalized patients have been shown to develop MERS-CoV antibody responses after 3 weeks,Reference Park, Perera and Choe 30 MERS-CoV antibody kinetics over time are not fully understood, particularly in asymptomatic or mildly ill individuals. Genome sequencing was limited by sample quality, and full-genome sequences were not available from all patient samples. HCP PPE use was assessed via interview, so errors in recollection may have been incorporated into our data. Due to the retrospective nature of this investigation, IPC practices during potential transmission events could not be confirmed by observation.
The introduction of MERS-CoV into healthcare facilities continues to occur, resulting in substantial morbidity and mortality. In these 2 contemporaneous but epidemiologically unrelated outbreaks, superspreading events were associated with extensive transmission and disruptions to hospital operations, including large-scale furloughing of exposed HCP. Early recognition of cases, rapid implementation of recommended IPC measures, and aggressive contact tracing and repeated testing are necessary to effectively prevent and interrupt transmission of MERS-CoV. (Fig. 4)
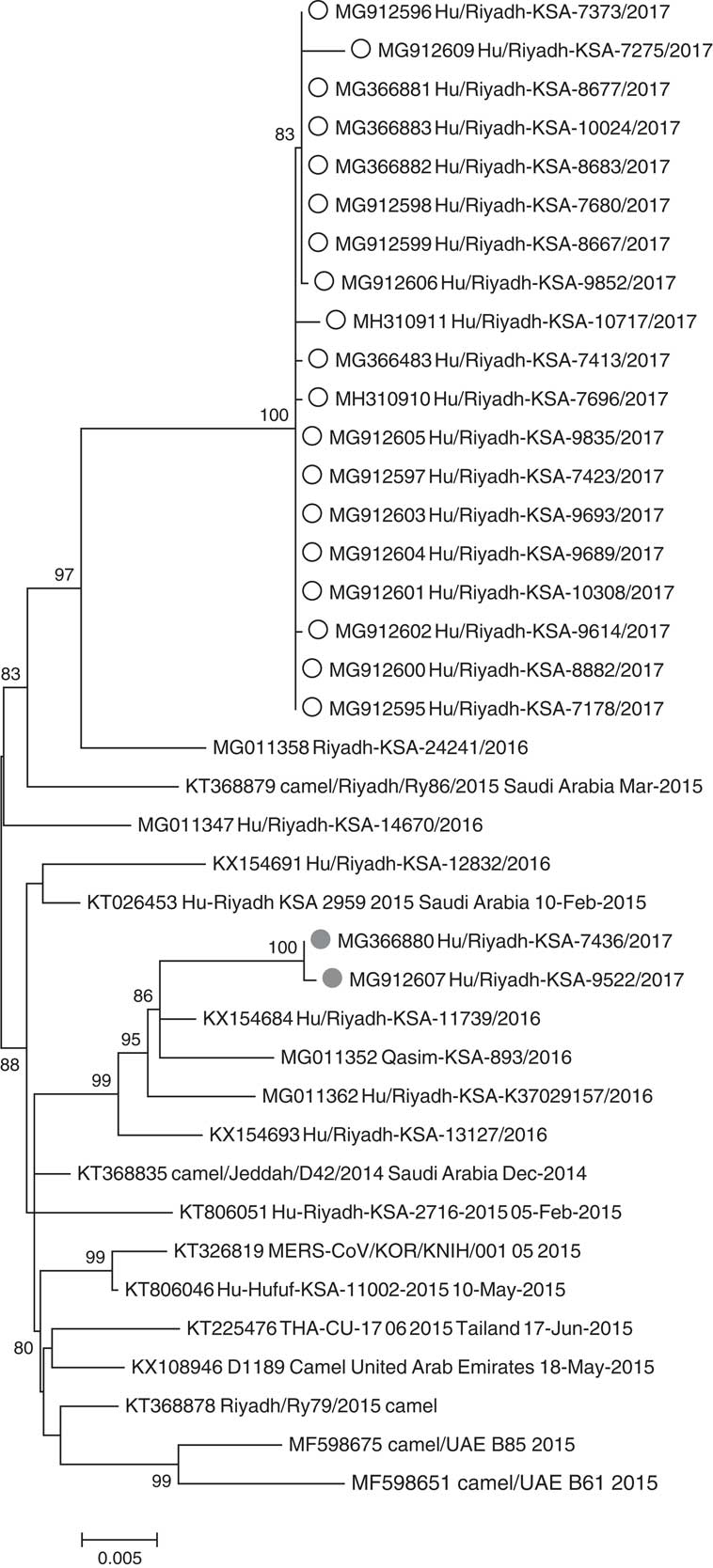
Fig. 4 Phylogenetic tree of MERS-CoV genomic sequences from this investigation and previously published sequences within clade 5. White circles indicate cases linked to hospital A, gray circles indicate cases linked to hospital B. All sequences have been deposited in GenBank.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.290











