Introduction
Clostridium difficile infection (CDI) is one of the most important healthcare-associated infections with high morbidity and mortality as well as healthcare costs (annually 8 billion dollars in the USA and 30 billion euros in Europe) [Reference Bouza1]. The costs of CDI in Korea also increased sharply, from US$2.4 million in 2008 to US$15.8 million in 2011 [Reference Choi2]. Metronidazole and vancomycin have been the mainstays of antibiotic treatment for CDI over the last 30 years. Clinical practice guidelines suggest that treatment should be chosen based on infection severity, with metronidazole being used for mild or moderate CDI and vancomycin for severe CDI [Reference Cohen3–Reference Surawicz5]. Factors associated with metronidazole failure include age older than 60 years, fever, hypoalbuminemia, peripheral leucocytosis, ICU stay and abnormal abdominal computed tomography (CT) imaging findings [Reference Belmares6–Reference Zar8]. Bauer et al. investigated the prognostic markers for severe CDI using the database of two randomised controlled trials and found that both leucocytosis and renal failure on the day of diagnosis were useful predictors of a complicated course of CDI [Reference Bauer9]. Other studies showed that age, ongoing treatment with systemic antibiotics, leukocyte count, albumin and serum creatinine can predict the risk of severe CDI [Reference Miller10, Reference Tedesco, Barton and Alpers11]. Recently, strain type has been suggested as an additional cause of excess morbidity, disease severity and high recurrence rates of CDI [Reference Miller12]. Accurate prediction of metronidazole failure, preferably early in the course of the disease, could shorten hospital stay and possibly reduce morbidity and mortality.
Since the administration of antibiotics is the most important causative factor of CDI, its initial management involves discontinuation of the offending antibiotic agent as soon as possible [Reference Cohen3–Reference Surawicz5]. However, the severity of the primary infection simply does not allow discontinuation of the antibiotic. Three previous small-scale studies showed that discontinuation of clindamycin successfully resolved the active symptoms of CDI [Reference Tedesco, Barton and Alpers11, Reference Gerding13, Reference Tedesco14]. A recent study investigated the effects of concomitant antibiotics on the response to fidaxomicin or vancomycin [Reference Mullane15]. Failure to stop the offending antibiotics is associated with decreased clinical cure rate and CDI recurrence [Reference Mullane15].
Therefore, we performed a retrospective study of patients who received metronidazole for the treatment of CDI over a 2-year period to investigate the predictors of treatment failure and the impact of the concomitant use of systemic antibiotics in these patients.
Methods
Study population and design
A retrospective cohort study was conducted among patients hospitalised at Samsung Changwon Hospital, a second care academic hospital, from January 2013 to December 2014. Eligible patients were identified by reviewing stool toxin enzyme immunoassay (EIA) results for C. difficile (Premier Toxins A&B, Meridian Bioscience) during the study period. Only patients who received metronidazole for ⩾3 days were included to evaluate the effect of metronidazole. The following information was collected: demographic characteristics, ward of acquisition, underlying comorbidities, recent medical history within 30 days of diagnosis of CDI, clinical presentations, laboratory parameters obtained 2 days before or 1 day after the diagnosis of CDI, concurrent infection and concomitant medication. To determine the severity of illness, McCabe classification was used for all patients [Reference McCabe16]. The study was approved by the institutional review board of Samsung Changwon Hospital. Informed consent was waived due to the observational retrospective nature of the study.
Definition
Diarrhoea was defined as the passage of at least three loose or watery stools within 24 h. CDI was defined as positive stool toxin EIA result in patients with diarrhoea. Treatment success was defined as the resolution of diarrhoea (⩽3 unformed stools for 48 h), improved parameters of disease severity (clinical, laboratory, radiological) and no new signs of severe disease development. Treatment failure was defined as an increase in diarrhoea or increased abdominal discomfort for more than 48 h, development of symptomatic ileus or toxic megacolon, persistent fever or recurrence of diarrhoea attributed to CDI while taking medication. A change in therapy was defined as a failure. Treatment response was checked daily and evaluated after at least 3 days. Concomitant use of antibiotics was regarded as the use of antibacterial agents for more than half of metronidazole's treatment duration. Concomitant antibiotics were further classified by the risk of contributing to the incidence or progression of CDI (high-risk, medium-risk and low-risk antibiotics) as previously described [Reference Mullane15]. Carbapenem, second-, third- or fourth-generation cephalosporin, fluoroquinolone, lincosamide, pivampicillin or temocillin were classified as high-risk antibiotics. Penicillin, penicillin combination, first-generation cephalosporin, macrolide, monobactam or streptogramin were classified as medium-risk antibiotics. All other systemic antibiotics were classified as low-risk antibiotics. Topical antibiotics and antifungal and antiviral agents with no antibacterial activity were not considered as concomitant antibiotics. Recurrence was defined as the reappearance of symptoms of CDI within 8 weeks after the onset of a previous episode; the presence of C. difficile toxin A, B or both in stool; and the need for retreatment.
Statistical analyses
Discrete data were presented as frequencies and percentages and continuous variables were summarised as the mean ± s.d. or as the median and interquartile range according to the distribution. Clinical, laboratory and therapeutic characteristics were compared between subgroups of treatment success and treatment failure using the χ 2 test, Fisher's exact test, two-sample t-test or Mann–Whitney U-test as appropriate. To identify the predictors of treatment failure, a multivariate logistic regression model was used to control for the effects of confounding variables. When the distribution of the continuous data was skewed, the log transformations of data were applied for univariate analyses. Variables with a P-value <0.05 in univariate analyses were candidates for multivariate analysis. All analyses were conducted with SPSS for Windows v.18.0 (SPSS Inc., Chicago, IL).
Results
A total of 377 patients with CDI were identified during the study period, of which 314 were enrolled in the study. Sixty-three patients were excluded from the analysis for the following reasons: patients receiving vancomycin only or combined with metronidazole (n = 14), cessation of the offending antibiotic agents (n = 19) and patients receiving metronidazole therapy <3 days (n = 30). Among the 314 patients with CDI receiving metronidazole therapy, 62 (19.7%) patients showed treatment failure. Thirty-three (53.2%) patients received concomitant antibiotics among the treatment failure group, while 72 (28.6%) patients received concomitant antibiotics among the treatment success group (Fig. 1).
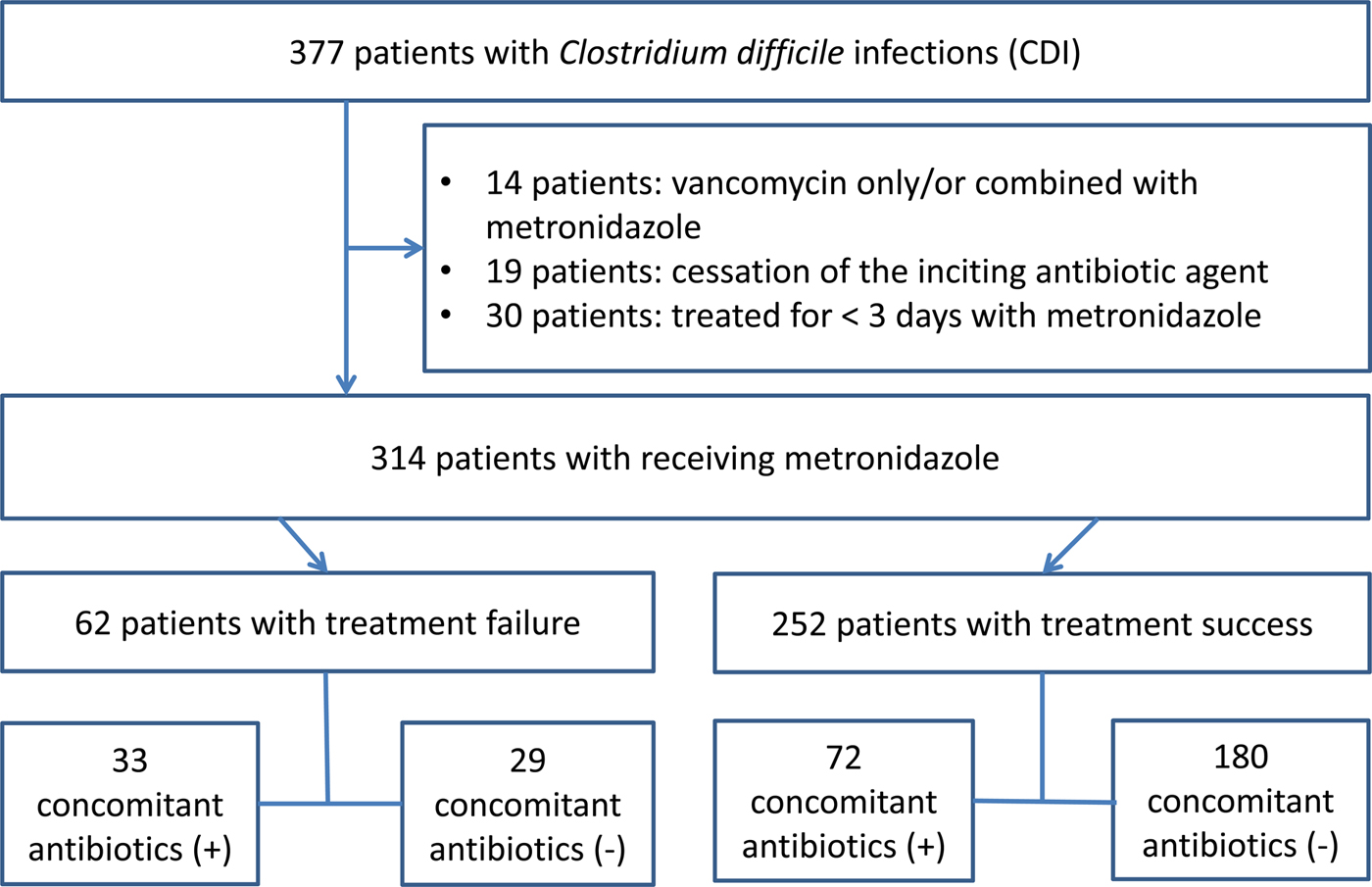
Fig. 1. Flowchart of study.
Comparison of characteristics between the treatment failure and treatment success groups
Among a total of 314 patients, patients aged ⩾65 were 62.21%. The most prevalent underlying disease was cerebrovascular diseases (43.6%), followed by diabetes (22.6%) and solid tumours (19.7%). A total of 276 (87.9%) patients had previous histories of antibiotic exposure within 30 days. Moreover, among 105 (33.4%) patients received concomitant antibiotics, 77 (73.3%) patients were treated with concomitant antibiotics with diagnoses of definitive infections. A total of 28 (26.7%) patients did not show any evidence of infection to needing concomitant antibiotics. Among patients receiving concomitant antibiotics (n = 105), 67.6% (n = 71) received high-risk antibiotics contributing to symptomatic CDI (Table 1).
Table 1. Characteristics between the treatment failure and treatment success groups following metronidazole treatment

ICU, intensive care unit; IQR, interquartile range; s.d., standard deviation; CRP, C-reactive protein; PPI, proton pump inhibitor.
Data are n (%) unless otherwise stated.
Clinical and laboratory characteristics of patients with treatment failure were compared with those of treatment success. Concomitant use of antibiotics had a significant effect on treatment failure (53.2% vs. 28.6%; P < 0.001). Underlying dialysis (19.4% vs. 6.0%; P = 0.001) and chronic renal failure without receiving dialysis (25.8% vs. 13.5%; P = 0.018), ultimate fatal underlying diseases (41.9% vs. 27.4%; P = 0.025) and indwelling central venous catheter (25.8% vs. 15.1%; P = 0.045) also significantly affected treatment failure. Other factors also included fever >38.3 °C (51.6% vs. 30.6%; P = 0.002) and presentation with septic shock (16.1% vs. 7.1%; P = 0.026). Regarding serological testing, low median serum albumin levels (2.30 vs. 2.65 g/dl; P < 0.001) had significant influences on treatment failure.
Predictors of treatment failure in CDI patients receiving metronidazole therapy
Multivariate analysis of potential risk factors associated with treatment failure is shown in Table 2. Variables with a P-value <0.05 in the univariate analysis were included in the subsequent multivariate analysis. A logistic regression model revealed that underlying dialysis (odds ratio (OR) 3.82, 95% confidence interval (CI) 1.03–14.10; P = 0.045)), fever >38.3 °C (OR 2.24, 95% CI 1.21–4.17; P = 0.011), low median serum albumin levels (OR 0.54, 95% CI 0.31–0.94; P = 0.028) and concomitant use of antibiotics (OR 3.22, 95% CI 1.50–6.92; P = 0.003) were independent predictors of treatment failure in patients with CDI receiving metronidazole therapy.
Table 2. Univariable and multivariable logistic regression analysis for independent risk factors for treatment failure in patients treated with metronidazole

OR, odds ratio; CI, confidence interval; ICU, intensive care unit; CRP, C-reactive protein.
*The log transformation of data was applied.
a Variables with a P-value of <0.05 in the univariate analyses were included in the subsequent multivariate regression model.
Hosmer and Lemeshow test, χ2 = 3.263, P = 0.917.
Outcomes of the concomitant antibiotic group
In the concomitant antibiotic group, treatment failure (31.4% vs. 13.9%; P < 0.001) and 30-day mortality (15.2% vs. 6.5%, P = 0.015) were more prevalent than those in the non-concomitant antibiotic group (Table 3). Although there was no difference between the two groups for recurrent CDI, there was a significant difference between the two groups if 12 patients from the non-concomitant antibiotic group receiving antibiotic treatment during the follow-up period were included to the concomitant antibiotic group (30.0% vs. 11.9%; P < 0.001).
Table 3. Outcomes of concomitant use of antibiotics during metronidazole treatment in patients with Clostridium difficile infections
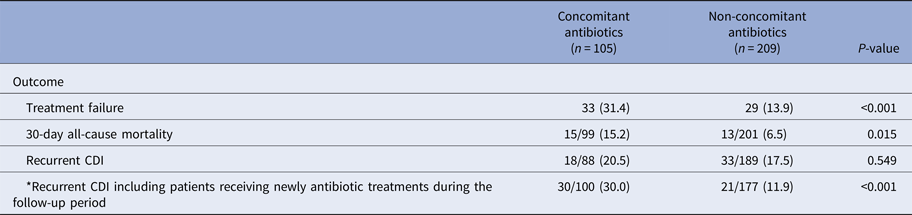
CDI, Clostridium difficile infection.
Data are n (%) unless otherwise stated.
*Twelve patients from the non-concomitant antibiotics group received antibiotic treatments during the follow-up period.
Table 4 summarises the outcomes of the concomitant antibiotic group by the risk of contributing to the incidence or progression of CDI. The rates of treatment failure, 30-day mortality and recurrent CDI were compared for patients receiving high-risk, medium-risk or low-risk antibiotics and those receiving no concomitant antibiotics. Only the concomitant use of high-risk antibiotics increased the rates of treatment failure (OR 3.59, 95% CI 1.93–6.68; P < 0.001) and 30-day mortality (OR 2.84, 95% CI 1.21–6.69; P = 0.017) when compared with the non-concomitant use of antibiotics. The concomitant use of high-risk antibiotics had no significant effect on recurrence, but there was a significant influence on recurrence if 12 patients from the non-concomitant antibiotic group receiving high-risk antibiotic treatments during the follow-up period were included to the concomitant antibiotic group (OR 3.83, 95% CI 1.96–7.47; P < 0.001).
Table 4. Effect of concomitant use of antibiotics on outcomes by risk of contributing to the incidence or progression of CDI
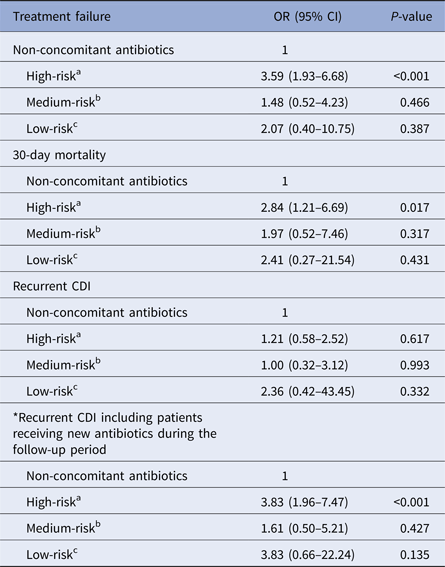
OR, odds ratio; CI, confidence interval; CDI, Clostridium difficile infection.
a High-risk antibiotic: carbapenem, second-, third- or fourth-generation cephalosporin, fluoroquinolone, lincosamide, pivampicillin or temocillin.
b Medium-risk antibiotic: penicillin, penicillin combination, first-generation cephalosporin, macrolide, monobactam or streptogramin.
c Low-risk antibiotic: all other systemic antibiotics.
*Twelve patients from the non-concomitant antibiotics group received high-risk antibiotic treatments during the follow-up period.
Discussion
This retrospective study showed that the treatment failure rate of patients with CDI receiving metronidazole treatment was 19.7%. Predictors of treatment failure were underlying dialysis, fever >38.3 °C, low median serum albumin levels, and concomitant antibiotics in patients with CDI receiving metronidazole treatment. A total of 33.4% of patients received concomitant antibiotics, of which 26.7% did not show any evidence of infection to needing concomitant antibiotics. The concomitant use of high-risk antibiotics increased the rates of treatment failure and 30-day mortality.
CDI still remains an important cause of morbidity and mortality in healthcare-associated infections [Reference Magill17]. Treatment strategies should be based on disease severity and risk of recurrence [Reference Cohen3–Reference Surawicz5]. For mild to moderate CDI, oral metronidazole remains the preferred therapy [Reference Cohen3–Reference Surawicz5]. However, a wide variety of risk factors for severe CDI have been suggested in the literature, which makes it difficult to set a rigid clinical prediction [Reference Cohen3–Reference Surawicz5, Reference Welfare18, Reference Abou Chakra, Pepin and Valiquette19]. Guidelines [Reference Cohen3–Reference Surawicz5] defined severe CDI as an episode with significant systemic toxin effects and shock, resulting in the need for ICU admission and colectomy or death. Therefore, one or more of the following clinical markers can be present: marked leucocytosis (leucocyte count >15 × 109/l), serum albumin of <3 g/dl, an increase in serum creatinine level of at least 1.5 times the premorbid level and severe underlying disease and/or immunodeficiency. In accordance with the guidelines’ suggestions, we found that fever >38.3 °C and low median serum albumin levels were associated with poor clinical outcome among patients with CDI receiving metronidazole therapy. These observations are a cause for concern as they indicate the poor adherence to clinical practice guidelines among healthcare providers. Previous studies suggested that adherence to the treatment guidelines was associated with a reduction in complications and mortality [Reference Brown and Seifert20, Reference Patel21]. Patients whose physicians followed the guidelines had a significant reduction in mortality (5.4% vs. 21.8%, P = 0.0012) [Reference Brown and Seifert20]. The findings from the above study and our research suggest that closer adherence to treatment guidelines may lead to better patient outcomes. Underlying dialysis was also a predictor of poor outcome among patients with CDI receiving metronidazole therapy, similar to that in previous studies, showing that patients with chronic kidney diseases undergoing long-term dialysis have longer treatment periods [Reference Thongprayoon22] and higher in-hospital morbidity [Reference Mullane23]. However, data on patients with chronic kidney disease and outcomes of CDI have generated inconsistent results. Therefore, guidelines have recognised only acute kidney injury as a marker of severe CDI [Reference Cohen3–Reference Surawicz5].
Guidelines recommended that any offending antimicrobial agent should be discontinued, if possible. A previous study [Reference Mullane15] showed that the use of concomitant antibiotics with CDI treatment was associated with a low initial response to CDI therapy and an extended time to resolution of diarrhoea. In the study, among 999 patients, 192 (19.2%) received concomitant antibiotics concurrently with vancomycin or fidaxomicin (days 1–10). In the absence of concomitant antibiotics, initial treatment failure was equivalent in both fidaxomicin and vancomycin (7.3% vs. 7.2%, P = 5.80). However, when patients received concomitant antibiotics with the study drug, those receiving vancomycin showed significantly higher treatment failure than those receiving fidaxomicin (20.6% vs. 10.0%, P = 0.04). In the present study, among the 377 patients with CDI receiving metronidazole therapy, a total of 33.4% received concomitant antibiotics. Initial treatment failure was noted in 13.9% of patients who did not receive concomitant antibiotics compared with 31.4% of those who received concomitant antibiotics concurrently with metronidazole. Compared with the previous study, patients receiving metronidazole therapy showed higher treatment failure rates both with and without concomitant antibiotics than those receiving vancomycin or fidaxomicin [Reference Johnson24]. Metronidazole has been recommended as the preferred treatment for mild or moderate CDIs, in part because of its low cost and reduced vancomycin-resistant enterococci (VRE) selection risk (2–4). However, CDI leads to increased VRE colonisation and/or VRE-related complications [Reference Poduval25]. Data also suggest that the prevalence of VRE is the same in both vancomycin- and metronidazole-treated CDI patients [Reference Al-Nassir26]. In addition, a recent systematic literature review indicated that metronidazole was cost-effective in only one of five economic evaluations when the analysis was restricted to data published in full manuscripts only [Reference Burton, Mitchell and Watt27]. In light of consistent observational evidence that showed a lower clinical success rate and vague cost-effectiveness for metronidazole vs. vancomycin [Reference Patel21, Reference Johnson24, Reference Burton, Mitchell and Watt27], it may be reasonable to consider vancomycin for mild-to-moderate CDI. Intriguingly, among patients receiving concomitant antibiotics, 26.7% did not show any evidence of infection to need the concomitant use of antibiotics. Therefore, exposure to antibiotics other than those intended for CDI should be avoided unless absolutely indicated. The significance of these observations cannot be overemphasised because the concurrent use of antibiotics is associated with increased treatment failure and mortality in patients with CDI receiving metronidazole therapy.
There was no significant relationship between concomitant antibiotic use during CDI treatment and recurrent CDI. However, concomitant antibiotic use was significantly associated with recurrent CDI if non-CDI antibiotic use both during and after CDI treatment was defined as the concomitant group. Non-CDI antibiotic use occurred after completion of CDI therapy in 12 patients. These 12 patients had more severe underlying diseases and longer hospital stays (data not shown). Consistent with this finding, a previous retrospective review of 246 patients showed an independent association of non-CDI antimicrobial use with recurrence but only when non-CDI antimicrobials were given after CDI therapy was completed [Reference Drekonja28].
The present study has some limitations. First, it was retrospective in design and observational. Thus, there is a risk of unmeasured confounding effects. Second, we did not investigate the strain type. The strain type has been suggested as an additional cause of excess morbidity, disease severity and higher recurrence rates of CDI [Reference Miller12]. However, hypervirulent strains of ribotype 027 were not common in Korean hospitals; ribotypes 018, 017 and 014/020 of C. difficile were the most prevalent in Korea [Reference Kim29]. Third, EIA demonstrated suboptimal sensitivity compared with the gold-standard cytotoxicity assay, which may have resulted in missing a substantial number of cases.
In conclusion, underlying dialysis, fever >38.3 °C, low median serum albumin levels and concomitant use of antibiotics were found to be independent predictors of treatment failure in patients with CDI receiving metronidazole treatment. Given the increasing recognition of the lack of response to treatment using metronidazole, the risk factors identified in this study may assist in predicting which patients will benefit from initial treatment with metronidazole and help to choose alternatives for those who will not. These results also suggest that careful investigation about the need for concomitant antibiotics is required, especially in patients receiving high-risk concomitant antibiotics.
Conflict of interest
None.









