Article contents
Amoebicidal activity of Cassia angustifolia extract and its effect on Acanthamoeba triangularis autophagy-related gene expression at the transcriptional level
Published online by Cambridge University Press: 10 May 2021
Abstract
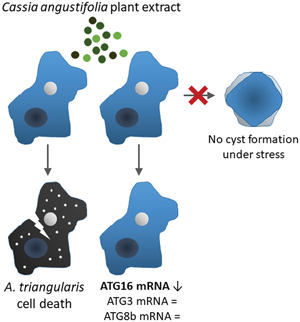
Cassia angustifolia Vahl. plant is used for many therapeutic purposes, for example, in people with constipation, skin diseases, including helminthic and parasitic infections. In our study, we demonstrated an amoebicidal activity of C. angustifolia extract against Acanthamoeba triangularis trophozoite at a micromolar level. Scanning electron microscopy (SEM) images displayed morphological changes in the Acanthamoeba trophozoite, which included the formation of pores in cell membrane and the membrane rupture. In addition to the amoebicidal activity, effects of the extract on surviving trophozoites were observed, which included cyst formation and vacuolization by a microscope and transcriptional expression of Acanthamoeba autophagy in response to the stress by quantitative polymerase chain reaction. Our data showed that the surviving trophozoites were not transformed into cysts and the trophozoite number with enlarged vacuole was not significantly different from that of untreated control. Molecular analysis data demonstrated that the mRNA expression of AcATG genes was slightly changed. Interestingly, AcATG16 decreased significantly at 12 h post treatment, which may indicate a transcriptional regulation by the extract or a balance of intracellular signalling pathways in response to the stress, whereas AcATG3 and AcATG8b remained unchanged. Altogether, these data reveal the anti-Acanthamoeba activity of C. angustifolia extract and the autophagic response in the surviving trophozoites under the plant extract pressure, along with data on the formation of cysts. These represent a promising plant for future drug development. However, further isolation and purification of an active compound and cytotoxicity against human cells are needed, including a study on the autophagic response at the protein level.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
References
- 5
- Cited by





