OVERVIEW OF THE CHALLENGES
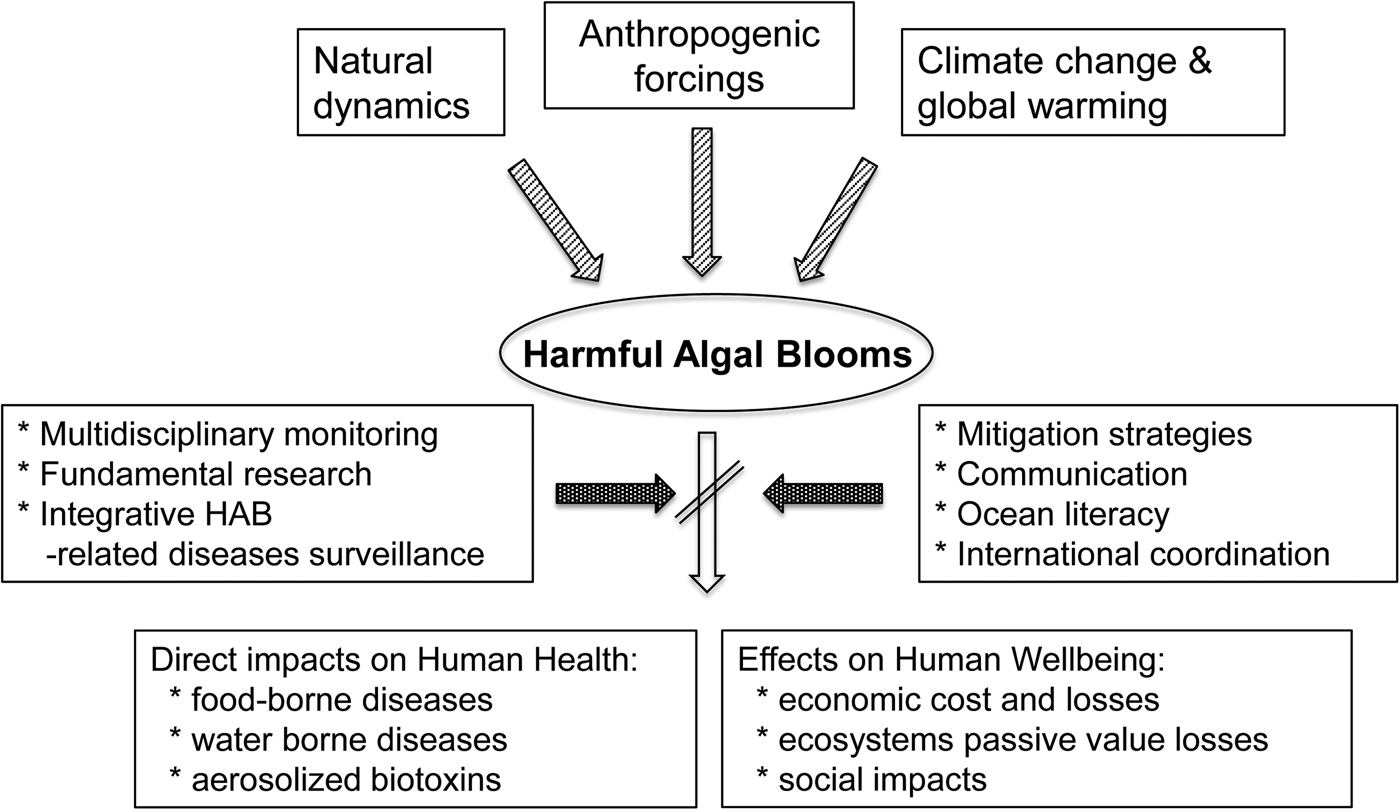
Aquatic ecosystems are supported by photosynthetic organisms (e.g. macrophytes, benthic and planktonic microalgae and cyanobacteria) that fix carbon, produce oxygen, and constitute the base of food webs. Under certain circumstances, however, the abundance of some taxa can reach levels that may cause harm to humans and other organisms. These proliferations often are referred to as ‘harmful algal blooms’ (HABs), a term that includes a variety of species and consequences that humans perceive as adverse. HABs occur in all aquatic environments (e.g. freshwater, brackish and marine) and at all latitudes. In this paper, we focus specifically on the threat that blooms of harmful microalgae pose to the benefits (food supplies, economic activities, tourism and recreation) that the oceans and seas provide to human health and wellbeing (Figure 1).

Fig. 1. Conceptual links between the main drivers (natural dynamics, climate change and global warming and other anthropogenic forcings) involved in the occurrence of HABs, the main impacts of HABs on humans health and wellbeing, and some of the tools to decrease these effects.
Of the many thousands of microalgal species described, about 300 are involved in harmful events (see e.g. http://www.marinespecies.org/hab/index.php). More than 100 of these species, with no apparent physiological, phylogenetic or structural commonalities, produce potent and persistent natural toxins that can be harmful or even lethal to humans and animals (Sournia, Reference Sournia, Lassus, Arzul, Erard, Gentien and Marcaillou1995; Moestrup et al., Reference Moestrup, Akselman, Cronberg, Elbraechter, Fraga, Halim, Hansen, Hoppenrath, Larsen, Lundholm, Nguyen and Zingone2009). The chemically diverse compounds synthesized by toxic HABs species have been associated with different syndromes in humans (Box 1), and many may also adversely affect certain fish, seabirds, reptiles and marine mammals (Box 2).
In humans, toxicity is caused by the ingestion of contaminated seafood products (fish or shellfish), skin contact with toxin-contaminated water, or the inhalation of aerosolized toxins or noxious compounds. In the case of food-borne poisonings (Figure 2), HAB toxins are bio-concentrated, often without apparently harming the vector marine organism that ingested the toxin, and transferred up through the food web to humans. Toxic effects usually occur when the HAB species producing the toxin is present in high abundance, although seafood poisoning also can be caused by highly toxic microalgae at low abundances. In addition to the direct impacts on human health, these toxic outbreaks have associated consequences on other components of human wellbeing both in terms of their socio-economic impact and costs. Namely, HAB occurrences can lead to the closure of important shellfisheries (e.g. Jin et al., Reference Jin, Thunberg and Hoagland2008) and increases in the costs of monitoring and management (Hoagland et al., Reference Hoagland, Anderson, Kaoru and White2002).

Fig. 2. Biotoxin transfer pathways through the marine food web to humans. A biotoxin-producing organism, such as the dinoflagellates Dinophysis acuta or Alexandrium catenella, is bioaccumulated by shellfish, which are apparently not affected by saxitoxin or lipophilic biotoxins. Consumption of the contaminated shellfish is a traditional way of diarrhetic or paralytic poisoning (DSP, PSP). Alternatively, some toxicogenic species attach to surfaces (macrophytes, corals) by an endogenous mucus (e.g. Gambierdiscus, Ostreopsis, Prorocentrum lima). Fragments of corals or macrophytes covered by the microalgae enter the food web through ingestion by herbivorous fish. This is the transmission mechanism of ciguatera fish poisoning (CFP). Certain fishes can also experience some sort of poisoning.
Another hazardous effect of some HABs taxa is the production of excess algal biomass, which can affect individual organisms and ecosystems in different ways (Box 2). When large blooms decay, the subsequent degradation by bacteria of accumulated biomass reduces oxygen concentrations in marine waters and can cause hypoxia, especially in bottom waters isolated by density gradients from surface waters. In addition to the benthic faunal mortalities related to oxygen depletion, the unpleasant appearance of surface scums and bad odours associated with some HABs can dissuade tourists from visiting coastal recreation areas (Scatasta et al., Reference Scatasta, Stolte, Granéli and van Ierland2003). Phycotoxins also can cause morbidities and mortalities of wild and farmed fish (gill damage), birds (hypothermia), marine mammals, or certain invertebrates, resulting in economic losses in finfish aquaculture and tourism, and losses of the non-market, ‘passive’ values that humans may have for protected species or extraordinary ecosystems. These examples illustrate how HABs may also decrease the non-market, passive use values of marine ecosystems and their services, thereby limiting the way in which the marine environment can enhance the quality of life and wellbeing for humans (Hoagland & Scatasta, Reference Hoagland, Scatasta, Granéli and Turner2006).
Blooms of microalgae, including those deemed by humans to be harmful (see for instance, http://haedat.iode.org), are a natural phenomenon (e.g. Smayda, Reference Smayda1997; Margalef, Reference Margalef, Reguera, Blanco, Fernández and Wyatt1998; Gowen et al., Reference Gowen, Tett, Bresnan, Davidson, McKinney, Harrison, Milligan, Mills, Silke and Crooks2012 and references cited therein) and awareness of HAB events is embedded in the cultural heritage of many coastal human communities. Historically, this awareness has helped to mitigate some of the adverse effects of HABs. However, at present, increasing the information about public health risks and the strategies that scientists and policy makers, working together, can address to decrease the impacts of HABs on human health and wellbeing could still be beneficial. It is important to recognize that there is not a realistic way to prevent HABs occurrence as it results from complex interactions among physical, chemical, and biological processes operating at different spatio-temporal scales in the marine environment. Over the last 50 years, human modifications of the marine environment, particularly those occurring at the land-sea interface, may have influenced the incidence of HABs in certain locations. These modifications include the alteration of water circulation in harbours and artificial beach construction, the dispersal of species through ship ballast waters, and nutrient enrichment (Hallegraeff & Bolch, Reference Hallegraeff and Bolch1992; Anderson et al., Reference Anderson, Glibert and Burkholder2002; Davidson et al., Reference Davidson, Gowen, Harrison, Fleming, Hoagland and Moschonas2014).
Superimposed on these stresses, climate change is already leading to temperature increases in some areas of the earth's oceans, and warmer waters could affect the occurrence of HABs (Moore et al., Reference Moore, Trainer, Mantua, Parker, Laws, Backer and Fleming2008; Backer & Moore, Reference Backer, Moore and El Nemr2011; Hallegraeff, Reference Hallegraeff2010 and references cited therein; Gowen et al., Reference Gowen, Tett, Bresnan, Davidson, McKinney, Harrison, Milligan, Mills, Silke and Crooks2012). Specifically, changes in the frequency, intensity, and geographic extent of HABs may occur, but the possible responses are likely to be highly species-specific, given the diverse toxicity, physiology, biology, and ecology of HAB organisms. Thus, the forecasting of such changes is still quite speculative, requiring long time series of ecological processes, as well as more focused research (including modelling).
This review was stimulated by discussions at the ‘Oceans and Human Health at the beginning of the 21st century’ workshop held in Bedruthan (Cornwall, UK) in March 2014. This paper is not an exhaustive review of all the different factors concerning the occurrence of HAB events (see e.g. GEOHAB, Reference Glibert and Pitcher2001, Reference Pitcher, Moita, Trainer, Kudela, Figueiras and Probyn2005, Reference Glibert2006, Reference Gentien, Reguera, Yamazaki, Fernand, Berdalet and Raine2008, Reference Furuya, Glibert, Zhou and Raine2010, Reference Berdalet, Tester and Zingone2012; Zingone & Wyatt, Reference Zingone, Wyatt, Robinson and Brinks2005; Gowen et al., Reference Gowen, Tett, Bresnan, Davidson, McKinney, Harrison, Milligan, Mills, Silke and Crooks2012). Instead, we summarize the main direct impacts of HABs on human health. We describe briefly the influences of HABs on human wellbeing, mainly through the negative consequences to ecosystem services and other marine organisms and environments. Other aspects of the possible interconnections between human wellbeing and HABs have yet to be investigated. Based on this evidence, we highlight the main challenges posed by marine HABs, and we discuss the tools available to respond to HABs in the coming years, especially within the context of climate warming.
DIRECT IMPACTS OF HABS ON HUMAN HEALTH
As noted above, the direct impacts of HABs in marine waters on human health are linked to poisoning (Box 1) associated with eating contaminated seafood (Figure 2), skin contact with contaminated water, and/or inhaling aerosolized biotoxins. In addition to human health conditions associated with known toxins produced by microalgae, there exist emerging phycotoxins and risks of poisoning through biotoxin contaminated desalinated drinking water. In the remaining text, the terms ‘biotoxin’, ‘phycotoxin’ or ‘toxin’ will be used to refer to toxic compounds synthesized by the marine microalgae.
Box 1. Main HAB toxic syndromes (in alphabetical order), Biotoxins (T), Causative Organisms (O), Symptoms (S), Route of exposure (E), Main Geographic Affected Areas (A), some References (R).
*Amnesic Shellfish Poisoning (ASP):
T: Domoic acid and isomers
O:Pseudo-nitzschia spp. and Nitzschia
S: Nausea, vomiting, diarrhoea, headache, dizziness, confusion, disorientation, short-term memory deficits, and motor weakness. Severe cases result in seizures, cardiac arrhythmia, respiratory distress, coma, and possibly death
E: Consumption of shellfish (possibly, fish)
A: Worldwide, affecting seafood and fisheries activities
R: Bates et al. (Reference Bates, Bird, de Freitas, Foxall, Gilgan, Hanic, Johnson, McCulloch, Odense, Pocklingtonm, Quilliam, Sim, Smith, Subba Rao, Todd, Walter and Wright1989); Martin et al. (Reference Martin, Haya, Wildish, Smayda and Shimizu1993); Scholin et al. (Reference Scholin, Gulland, Doucette, Benson, Busman, Chavez, Cordaro, DeLong, De Vogelaere, Harvey, Haulena, Lefebvre, Lipscomb and Van Dolah2000); Fehling et al. (Reference Fehling, Davidson, Bolch and Bates2004)
*Azaspiracid Shellfish Poisoning (AZP):
T: Azaspiracid and its derivatives
O: Amphidomataceae (Amphidoma languida, Azadinium spinosum, Azadinium poporum, Azadinium dexteroporum)
S: Nausea, vomiting, severe diarrhoea, abdominal cramps; effects on mice tests include severe damage to the intestine, spleen and liver tissues in animal tests
E: Consumption of shellfish
A: Seafood poisoning reported from shellfish in Europe and North America
R: Twiner et al. (Reference Twiner, Rehmann, Hess and Doucette2008, Reference Twiner, Doucette, Rasky, Huang, Roth and Sanguinetti2012a, Reference Twiner, Hanagriff, Butler, Madhkoor and Doucetteb, Reference Twiner, Hess, Doucette and Botana2014); Klontz et al. (Reference Klontz, Abraham, Plakas and Dickey2009); Tillmann et al. (Reference Tillmann, Elbrächter, Krock, John and Cembella2009, Reference Tillmann, Salas, Jauffrais, Hess, Silke and Botana2014); Hess et al. (Reference Hess, McCarron, Krock, Kilcoyne, Miles and Botana2014)
*Ciguatera Fish Poisoning (CFP):
T: Ciguatoxin
O:Gambierdiscus spp., Fukuyo spp.
S: Nausea, vomiting, diarrhoea, numbness of mouth and extremities. Neurological symptoms may persist for several months
E: Consumption of coral reef fish
A: Endemic in the tropics and subtropics, expanding to temperate latitudes
R: Friedman et al. (Reference Friedman, Fleming, Fernandez, Bienfang, Schrank, Dickey, Bottein, Backer, Ayyar, Weisman, Watkins, Granade and Reich2008); Litaker et al. (Reference Litaker, Vandersea, Faust, Kibler, Nau, Holland, Chinain, Holmes and Tester2010); Chinain et al. (Reference Chinain, Darius, Ung, Cruchet, Wang, Ponton, Laurent and Pauillac2010a, Reference Chinain, Darius, Ung, Fouc, Revel, Cruchet, Pauillac and Laurentb); Tester et al. (Reference Tester, Kibler, Holland, Usup, Vandersea, Leaw, Lim, Jacob Larsen, Mohammad-Noor, Faust and Litaker2014)
*Diarrhetic Shellfish Poisoning (DSP):
T: Okadaic acid and its derivatives (dinophysistoxins)
O:Dinophysis spp., Prorocentrum lima
S: Nausea, vomiting, severe diarrhoea, abdominal cramps, respiratory distress
E: Consumption of shellfish
A: Worldwide, affecting seafood and fisheries activities
R: Yasumoto et al. (Reference Yasumoto, Sugawara, Fukuyo, Oguri, Igarashi and Fujita1980); Kat (Reference Kat1983); Reguera & Pizarro (Reference Reguera, Pizarro and Botana2008); Raine et al. (Reference Raine, McDermott, Silke, Lyons, Nolan and Cusack2010); Reguera et al. (Reference Reguera, Riobó, Rodríguez, Díaz, Pizarro, Paz, Franco and Blanco2014)
*Neurotoxic Shellfish Poisoning (NSP) and respiratory irritation:
T: Brevetoxins
O:Karenia brevis (predominantly)
S: By seafood poisoning: nausea, temperature sensation reversals, muscle weakness, and vertigo. Exposure to aerosols related to respiratory and eye irritation particularly for asthmatics
E: Consumption of shellfish (and fish at least for marine mammals); inhalation of marine aerosols during active blooms
A: Particularly in the Gulf of Mexico and Japan, China, Korea, New Zealand
R: Watkins et al. (Reference Watkins, Reich, Fleming and Hammond2008); Fleming et al. (Reference Fleming, Kirkpatrick, Backer, Walsh, Nierenberg, Clark, Reich, Hollenbeck, Benson, Cheng, Naar, Pierce, Bourdelais, Abraham, Kirkpatrick, Zaias, Wanner, Mendes, Shalat, Hoagland, Stephan, Bean, Watkins, Clarke, Byrne and Baden2011)
*Palytoxicosis (foodborne poisoning) and other irritative symptoms:
T: Palytoxin, Ostreocin, Ovatotoxin
O: Ostreopsis spp.
S: Associated to food-borne poisoning: nausea, vomiting, severe diarrhoea, abdominal cramps, lethargy, tingling of the lips, mouth, face and neck, lowered heart rate, skeletal muscle breakdown, muscle spasms and pain, lack of sensation, myalgia and weakness, hypersalivation, difficulty in breathing. Exposure to aerosols: eye and nose irritation, whinorrhoea, general malaise, fever. Cutaneous irritations in beach swimmers
E: Consumption of seafood; inhalation of marine aerosols; direct contact with water A: Food-borne poisoning in the tropics and subtropics; respiratory and cutaneous irritations in Mediterranean beaches
R: Deeds & Schwartz (Reference Deeds and Schwartz2010) (references therein); Tubaro et al. (Reference Tubaro, Durando, Del Favero, Ansaldi, Icardi and Deeds2011); Ciminiello et al. (Reference Ciminiello, Dell'Aversano, Dello Iacovo, Fattorusso, Forino, Grauso, Tartaglione, Guerrini and Pistocchi2010, Reference Ciminiello, Dell'Aversano, Dello Iacovo, Fattorusso, Forino, Tartaglione, Yasumoto, Battocchi, Giacobbe, Amorim and Penna2013); Vila et al. (Reference Vila, Arin, Battocchi, Bravo, Fraga, Penna, Reñé, Riobó, Rodríguez, Sala, Camp, de Torres and Franco2012)
*Paralytic Shellfish Poisoning (PSP):
T: Saxitoxin and derivatives
O: Alexandrium spp., Pyrodinium bahamense var. compressum and other species, Gymnodinium catenatum, some calcareous red macroalgae
S: Nausea, vomiting, diarrhoea, numbness and tingling of the lips, mouth, face and neck. Severe cases can result in paralysis of the muscles of the chest and abdomen leading to death
E: Consumption of shellfish, crustaceans, fish
A: Worldwide, affecting shellfish activities
R: Ayres (Reference Ayres1975); Gaines & Taylor (Reference Gaines, Taylor, Anderson, White and Baden1985); Anderson et al. (Reference Anderson, Sullivan and Reguera1989, Reference Anderson, Keafer, Geyer, Signell and Loder2005a, Reference Anderson, Stock, Keafer, Nelson, McGillicuddy, Keller, Thompson, Matrai and Martinb)
Food-borne diseases: overview
Poisoning through the ingestion of biotoxin-contaminated seafood is the best documented impact that HABs have on humans (Figure 2, Box 1). The poisoning process involves the bio-concentration of the biotoxins by filter feeding fauna (mostly bivalve molluscs, e.g. Mytilus spp.) which themselves are generally unaffected by these compounds. Other vectors include certain marine gastropods (e.g. whelks and moon snails), some crustaceans (e.g. crabs), echinoderms and fish (e.g. some planktivorous fishes or belonging to the tetraodontidae family) that acquire biotoxins through the food web (Deeds et al., Reference Deeds, Landsberg, Etheridge, Pitcher and Longan2008). Toxins accumulated in seafood tissues can remain for considerable lengths of time after the bloom has declined in the seawater. Further, these biotoxins are not destroyed by cooking or by the processing of seafood products, and because they do not have distinctive odours or taste, they can be detected only through specialized laboratory testing (Zaias et al., Reference Zaias, Backer, Fleming, Rabinowitz and Conti2010).
Most algal toxins are primarily neurotoxins (e.g. brevetoxins affecting the Na+ channels), although they are also known to affect human health through other routes (e.g. okadaic acid affecting phosphatase activity). The various toxic compounds can produce a wide range of symptoms and thus have been associated with several clinically described syndromes depending on the main symptomatic mode (Box 1): amnesic (ASP), azaspirazid (AZP), diarrhetic (DSP), neurotoxic (NSP) and paralytic (PSP) shellfish poisonings and ciguatera fish poisoning (CFP). The syndromes can present with symptoms from the nervous, digestive, respiratory, hepatic, dermatological or cardiac systems (e.g. Baden & Trainer, Reference Baden, Trainer and Falconer1993). The effects can be acute (e.g. paralytic shellfish poisoning can occur within minutes to hours; Medcof, Reference Medcof, Anderson, White and Baden1985) and can last for weeks to months (e.g. ciguatera fish poisoning; Friedman et al., Reference Friedman, Fleming, Fernandez, Bienfang, Schrank, Dickey, Bottein, Backer, Ayyar, Weisman, Watkins, Granade and Reich2008). Although there is considerable knowledge on the acute health effects caused by HAB biotoxins, many of the toxicological mechanisms are incompletely understood. In addition, we know little about the chronic effects of these biotoxins, either from acute exposure that produces long-lasting damage, or from chronic low-level exposures over long periods of time.
For the food-borne syndromes, the prevention of contaminated shellfish reaching the markets (by monitoring the causative species and/or the presence of biotoxin in seafood in real time) is currently the only effective way to protect human health. In fact, well-structured monitoring programmes targeting the causative organisms and toxins in commercial seafood associated with the clinical syndromes (i.e. ASP, AZP, DSP, NSP and PSP) have proven effective in reducing the human exposure to biotoxins in many areas of the world. Information about operative HAB monitoring programmes is not compiled at a worldwide scale, only at regional as for the North Atlantic by the ICES (International Council for the Exploration of the Sea) – IOC UNESCO Working Group on Harmful Algal Blooms Dynamics (http://www.ices.dk/community/groups/Pages/WGHABD.aspx). However, monitoring could be enhanced by complementing it with additional actions such as informing the public in non-commercial areas affected by HABs (e.g. Reich et al., Reference Reich, Lazensky, Faris, Fleming, Kirkpatrick, Watkins, Ullman, Kohler and Hoagland2015) and increasing the understanding of the complex processes involved in harmful events (e.g. Whyte et al., Reference Whyte, Swan and Davidson2014).
Unfortunately, due to increased human pressure on coastal marine ecosystems together with global warming, harmful blooms may occur in areas where they have not previously been reported (Trainer et al., Reference Trainer, Moore, Bill, Adams, Harrington, Borchert, Silva and Eberhart2013). Also, new biotoxins are continually being identified. For these cases, toxin detection and identification of the causative organism represent new challenges for monitoring and management procedures (Turner et al., Reference Turner, Higgins, Veszelovski, Payne, Davidson, Hungerford and Higman2015). It is also noteworthy that for ciguatera fish poisoning, the most frequent cause of HAB-associated poisoning in tropical waters, effective procedures to protect human populations are lacking (see Sections ‘Most common biotoxin syndromes in temperate latitudes’ and ‘Better quantification and prevention of the impacts on human health: HAB-related disease surveillance’). Increased international tourist travel and trade in seafood can lead to poisonings in areas far from where the fish is caught (Mattei et al., Reference Mattei, Vetter, Eisenblätter, Krock, Ebbecke, Desel and Zimmermann2014).
MOST COMMON BIOTOXIN SYNDROMES IN TEMPERATE LATITUDES
In the temperate latitudes of Europe, South Africa, Asia, Australia, North America and South America, the most common HABs cause amnesic (ASP), azaspiracid (AZP), diarrhetic (DSP), neurotoxic (NSP) and paralytic (PSP) shellfish poisonings (Box 1).
Domoic acid, a neurotoxin produced by various species of Pseudo-nitzschia and Nitzschia, was identified as responsible for causing an outbreak of amnesic shellfish poisoning in humans (involving 107 illnesses and three deaths) after the consumption of blue mussels from Prince Edward Island (Canada) in 1987 (Bates et al., Reference Bates, Bird, de Freitas, Foxall, Gilgan, Hanic, Johnson, McCulloch, Odense, Pocklingtonm, Quilliam, Sim, Smith, Subba Rao, Todd, Walter and Wright1989; Todd, Reference Todd1993). Since then, blooms of these pennate diatoms have resulted in a range of, often large-scale, shellfish toxicity events, affecting humans and other large vertebrates (see Section ‘Impacts of HABs on non-market, passive use values of marine ecosystems’ and Box 2). Symptoms of ASP poisoning in humans include short- and long-term memory loss.
The azaspiracids, first identified in mussels from Ireland in 1995 (Satake et al., Reference Satake, Ofuji, Naoki, James, Furey, McMahon, Silke and Yasumoto1998), belong to a novel group of polyether biotoxins produced by the small armoured dinoflagellate Azadinium spinosum (Tillmann et al., Reference Tillmann, Elbrächter, Krock, John and Cembella2009; see also Section ‘Improving monitoring and research needs to forecast and predict HAB events’). This biotoxin causes symptoms similar to those displayed by DSP (Twiner et al., Reference Twiner, Rehmann, Hess and Doucette2008), although slowly progressing paralyses have also been observed in mouse assays. Azaspiracids have now been found in a number of other European Union countries with ~20 different analogues of AZA identified.
Diarrhetic shellfish poisoning (DSP) was first linked to the presence of Dinophysis fortii in Japan (Yasumoto et al., Reference Yasumoto, Sugawara, Fukuyo, Oguri, Igarashi and Fujita1980) and to D. acuminata in Dutch coastal waters (Kat, Reference Kat1983), and it was recorded after consumption of mussels containing DSP biotoxins from the Northern Adriatic coast in 1989 (Boni et al., Reference Boni, Mancini, Milandri, Poletti, Pompei, Viviani, Vollenweider, Marchetti and Viviani1992). Diarrhetic shellfish poisoning is caused by okadaic acid or its derivative dinophysistoxins, produced by 10 species of the genus Dinophysis, two species of the genus Phalachroma (Reguera et al., Reference Reguera, Velo-Suárez, Raine and Park2012), and Prorocentrum lima (Koike et al., Reference Koike, Sato, Yamaji, Nagahama, Kotaki, Ogata and Kodama1998). In humans, DSP biotoxins bind to phosphatase receptors, causing severe, but not usually fatal, gastrointestinal symptoms (with a rapid onset). DSP outbreaks are common in Europe, affecting shellfish consumers in at least 10 countries. On occasion, outbreaks have resulted in large numbers of people becoming ill, and shellfish harvest areas have been closed for up to 10 months (e.g. Fraga & Sánchez, Reference Fraga, Sánchez, Anderson, White and Baden1985; Haamer et al., Reference Haamer, Andersson, Lindahl, Lange, Li and Edebo1990; Lassus et al., Reference Lassus, Bardouil, Truquet, Le Baut, Pierre, Anderson, White and Baden1985; Ramstad et al., Reference Ramstad, Hovgaard, Yasumoto, Larsen and Aune2001; Blanco et al., Reference Blanco, Moroño and Fernández2005, Reference Blanco, Correa, Muñíz, Mariño, Martín and Arévalo2013; Vale et al., Reference Vale, Botelho, Rodrigues, Gomes and Sampayo2008; review by Reguera et al., Reference Reguera, Riobó, Rodríguez, Díaz, Pizarro, Paz, Franco and Blanco2014).
For regulatory purposes, the more recently discovered pectenotoxins and yessotoxins are classified within the DSP group. Pectenotoxins are produced by some of the Dinophysis species including D. acuta and D. acuminata. Yessotoxins induce similar symptoms but are produced by the dinoflagellates Lingulodinium polyedrum and Protoceratium reticulatum (Paz et al., Reference Paz, Riobó, Fernández, Fraga and Franco2004 and references cited there in). Recently, experts have recommended the deregulation of pectenotoxins and yessotoxins due to research results finding a non-toxic effect of the oral administration of these substances in mice (ICES, 2006).
Brevetoxin is the collective name given to a class of biotoxins that causes neurotoxic shellfish poisoning (NSP). Brevetoxins are produced primarily by the naked dinoflagellate Karenia brevis. In the Gulf of Mexico, and in isolated instances along the South-east Atlantic coast of the USA, blooms of K. brevis have caused water discolouration, large-scale finfish mortality events, human poisonings due to the consumption of shellfish, and respiratory problems in asthmatics caused by inhalation of biotoxin in the form of an aerosol (Morris et al., Reference Morris, Campbell, Taylor and Freeman1991; Magaña et al., Reference Magaña, Contreras and Villareal2003; Kirkpatrick et al., Reference Kirkpatrick, Fleming, Squicciarini, Backer, Clark, Abraham, Benson, Cheng, Johnson, Pierce, Zaias, Bossart and Baden2004; Watkins et al., Reference Watkins, Reich, Fleming and Hammond2008; Fleming et al., Reference Fleming, Kirkpatrick, Backer, Walsh, Nierenberg, Clark, Reich, Hollenbeck, Benson, Cheng, Naar, Pierce, Bourdelais, Abraham, Kirkpatrick, Zaias, Wanner, Mendes, Shalat, Hoagland, Stephan, Bean, Watkins, Clarke, Byrne and Baden2011). Notably, NSP has not been linked to fatalities in humans (van Dolah, Reference Van Dolah2000). The occurrence of toxic K. brevis red tides was recorded as early as 1648 in the Western Gulf of Mexico and since the 1840s in Florida (Magaña et al., Reference Magaña, Contreras and Villareal2003; Kirkpatrick et al., Reference Kirkpatrick, Fleming, Squicciarini, Backer, Clark, Abraham, Benson, Cheng, Johnson, Pierce, Zaias, Bossart and Baden2004).
Paralytic shellfish poisoning (PSP) is caused by saxitoxin and its derivatives, potent neurotoxins that can cause headache, nausea, facial numbness, and, in severe cases, respiratory failure and death. The first likely cases in the UK were in 1827 in Leith and in 1888 in Liverpool (Ayres, Reference Ayres1975). In British Columbia (Gaines & Taylor, Reference Gaines, Taylor, Anderson, White and Baden1985) and Norway (Yndestad & Underdal, Reference Yndestad, Underdal, Anderson, White and Baden1985), the first recorded outbreaks of PSP were in 1793 and 1901 respectively. Medcof (Reference Medcof, Anderson, White and Baden1985) reported toxic shellfish episodes from the 1930s and 1940s. In Europe and North America, PSP is mainly associated with blooms of the thecate (armoured) dinoflagellate genus Alexandrium, mainly A. tamarense/funyense/catenella group and A. minutum (Medlin et al., Reference Medlin, Lange, Wellbrock, Donner, Elbrachter, Hummert and Luckas1998; Higman et al., Reference Higman, Stone and Lewis2001; Lilly et al., Reference Lilly, Halanych and Anderson2007; Touzet et al., Reference Touzet, Franco and Raine2007), although some Alexandrium species and strains are non-toxic. In Asia (the Philippines, Malaysia, Brunei and Indonesia, Papua New Guinea), PSP is mainly produced by outbreaks of the armoured dinoflagellate Pyrodinium bahamense var. compressum. In the Philippines, this species was responsible for 1995 cases with 117 deaths linked to PSP toxicity between 1983 and 1999 (Azanza, Reference Azanza1999; Azanza & Taylor, Reference Azanza and Taylor2001).
CIGUATERA FISH POISONING (CFP)
Worldwide, ciguatera fish poisoning (CFP) is the most common food poisoning associated with a natural, non-bacterial chemical, and it occurs throughout the tropics, particularly in vulnerable island communities such as the Pacific Island Countries and Territories (PICTs). Ciguatoxins, or their precursors, are produced by several species of the benthic dinoflagellate genus Gambierdiscus (mainly G. toxicus). The biotoxins are modified through metabolic pathways in the food web of coral reefs, and they tend to accumulate in fish, particularly larger carnivorous species such as barracuda (Sphyraena spp., Heymann, Reference Heymann2004), but they also have been found in more than 400 fish species. After consuming ciguatoxin-contaminated fish, a range of acute neurological, gastrointestinal and cardiac symptoms have been reported, with some individuals experiencing chronic neurological symptoms lasting weeks to months (e.g. Freudenthal, Reference Freudenthal, Graneli, Sundstrom, Edler and Anderson1990; Friedman et al., Reference Friedman, Fleming, Fernandez, Bienfang, Schrank, Dickey, Bottein, Backer, Ayyar, Weisman, Watkins, Granade and Reich2008; Skinner et al., Reference Skinner, Brewer, Johnstone, Fleming and Lewis2011, and references cited therein). Repeated exposure to ciguatoxins can reportedly exacerbate the acute ciguatera symptoms (Bagnis et al., Reference Bagnis, Kuberski and Laugeir1979; Pottier et al., Reference Pottier, Vernoux and Lewis2001).
CFP is a threat to public health throughout tropical areas, and it is the most widespread, and hence best described, in the island nations of the Pacific and the Caribbean (for reviews, see Bruslé, Reference Bruslé1997; Lehane & Lewis, Reference Lehane and Lewis2000; Friedman et al., Reference Friedman, Fleming, Fernandez, Bienfang, Schrank, Dickey, Bottein, Backer, Ayyar, Weisman, Watkins, Granade and Reich2008; and references therein). Unfortunately, the real extent of illness is not well documented due to under-reporting and misdiagnosis (McKee et al., Reference McKee, Fleming, Tamer, Weisman, Hallegraeff, Blackburn, Bolch and Lewis2001; Radke et al., Reference Radke, Reich and Morris2015). Thus, its impacts on human communities and ecosystem health are still poorly understood. Data from the Health and Fisheries Authorities of 17 PICTs (Skinner et al., Reference Skinner, Brewer, Johnstone, Fleming and Lewis2011) estimated a mean annual incidence of 104 cases per 100,000 people across the region during 1973–83. Based on these estimates, the recorded CFP incidence in the South Pacific should be increased by 60%, i.e. up to 194 cases per 100,000 people between 1998–2008.
CFP illness rates exhibit high variability, reaching particularly elevated levels in some areas. For instance, up to 497 cases per 10,000 population per year were recorded in French Polynesia including Raivavae Island (Austral archipelago) between 2000–08 (Chateau-Degat et al., Reference Chateau-Degat, Chinain, Darius, Dewailly and Mallet2009; Chinain et al., Reference Chinain, Darius, Ung, Fouc, Revel, Cruchet, Pauillac and Laurent2010b), and up to 440 cases per 10,000 population per year in the Caribbean between 1996–2006 (Tester et al., Reference Tester, Feldman, Nau, Kibler and Litaker2010). Despite uncertainty about the real incidence, data suggest that CFP constitutes an acute and chronic illness with major public health significance at both local and more widespread levels. In future, the health problem could be exacerbated due to anthropogenic pressures, such as the increased development of coastal zones, ocean warming (e.g. Villareal et al., Reference Villareal, Hanson, Qualia, Jester, Granade and Dickey2007; Kibler et al., Reference Kibler, Tester, Kunkel, Moore and Litaker2015), or natural disasters, such as hurricanes. These pressures can lead to the damage of coral reefs, favouring more resilient macroalgae that constitute new surfaces for the proliferation of the involved toxicogenic benthic dinoflagellates (e.g. Chateau-Degat et al., Reference Chateau-Degat, Chinain, Darius, Dewailly and Mallet2005; Tester et al., Reference Tester, Feldman, Nau, Kibler and Litaker2010).
It is inherently difficult to cope with CFP as a public health problem. For example, the risks of CFP often have been traditionally ‘managed’ by native fishermen using their local, traditional knowledge, who may warn each other about areas where ciguatoxin-contaminated fish are known to occur. With increasing trade and coastal tourism, CFP cases could occur in non-tropical areas, where ciguatera often goes unrecognized or diagnosed only after expensive investigation (Epelboin et al., Reference Epelboin, Pérignon, Hossen, Vincent, Krys and Caumes2014; Mattei et al., Reference Mattei, Vetter, Eisenblätter, Krock, Ebbecke, Desel and Zimmermann2014). There is neither a reliable, cost-effective method for detecting the biotoxin nor is there a biomarker to diagnose the illness in humans. A review by Friedman et al. (Reference Friedman, Fleming, Fernandez, Bienfang, Schrank, Dickey, Bottein, Backer, Ayyar, Weisman, Watkins, Granade and Reich2008) describes the clinical course of CFP and its possible treatments, many of which may be unclear to other healthcare providers and public health practitioners, even in endemic areas.
POISONING BY PALYTOXIN AND ANALOGUES
Ostreopsis is a dinoflagellate genus co-occurring with Gambierdiscus. In tropical Indo-Pacific latitudes, Ostreopsis has been associated with poisoning after the ingestion of clupeid fishes, crabs or sea urchins contaminated with palytoxin (PLTX) (Noguchi et al., Reference Noguchi, Hwang, Arakawa, Daigo, Sato, Ozaki, Kawai, Gopalakrishnakone and Tan1987; Taniyama et al. Reference Taniyama, Arakawa, Terada, Nishio, Takatani, Mahmud and Noguci2003; see review by Deeds & Schwartz, Reference Deeds and Schwartz2010, and references cited therein). This compound and its analogues (Ciminiello et al., Reference Ciminiello, Dell'Aversano, Dello Iacovo, Fattorusso, Forino and Tartaglione2011, Reference Ciminiello, Dell'Aversano, Forino, Botana and Alfonso2015 and references therein; Brissard et al., Reference Brissard, Hervé, Sibat, Séchet, Hess, Amzil and Herrenknecht2015) are among the most potent biotoxins of marine origin. Nevertheless, in spite of the well-documented but very limited number of severe PLTX-related poisoning cases in these tropical areas, the true risk of PLTX poisoning through seafood consumption in humans is difficult to assess. This is due to the co-occurrence of PLTX with other seafood biotoxins (e.g. ciguatoxins, saxitoxins and tetrodotoxin), the distribution of PLTX mainly in the tropical areas of developing nations with little surveillance and reporting, and its prevalence in reef species that are not commercially harvested.
Ostreopsis seems to be expanding to temperate latitudes (Rhodes, Reference Rhodes2011), and recurrent blooms have occurred in Mediterranean waters during the last two decades (e.g. Mangialajo et al., Reference Mangialajo, Ganzin, Accoroni, Asnaghi, Blanfune, Cabrini, Cattaneo-Vietti, Chavanon, Chiantore, Cohu, Costa, Fornasaro, Grossel, Marco-Miralles, Masó, Reñé, Rossi, Sala, Thibaut, Totti, Vila and Lemée2011; Illoul et al., Reference Illoul, Rodríguez, Vila, Adjas, Aït Younes, Bournissa, Koroghli, Marouf, Rabia and Ameur2012). In this region, Ostreopsis proliferations have been related to aerosol exposures at bathing beaches (see Section ‘Ostreopsis cf. ovata and respiratory irritations’) and macrofaunal mortalities (Box 2). Fortunately, to date, food poisonings related to the PLTX-like group have not been reported in the Mediterranean, although the biotoxin has been detected in certain marine fauna (e.g. Aligizaki et al., Reference Aligizaki, Katikou, Milandri and Diogène2011; Biré et al., Reference Biré, Trotereau, Lemée, Delpont, Chabot, Aumond and Krys2013; Brissard et al., Reference Brissard, Herrenknecht, Séchet, Hervé, Pisapia, Harcouet, Lémée, Chomérat, Hess and Amzil2014; Ciminiello et al., Reference Ciminiello, Dell'Aversano, Forino, Botana and Alfonso2015). The European regulation to monitor PLTX has not yet become established, although the Panel on Contaminants in the Food Chain (CONTAM Panel) of the European Food Safety Authority (EFSA, 2009) assessed the risks to human health associated with the presence of PLTX-group biotoxins in shellfish, recommending a maximum concentration of 30 µg eqPLTX kg−1 fresh weight. Overall, the few available data suggest a growing potential risk of seafood contamination (from commercial or recreational fishing or aquaculture) in Mediterranean coastal waters (especially in those affected by recurrent Ostreopsis blooms).
The possible transfer and accumulation of the PLTX-like group of biotoxins through the food web to humans should be investigated further. Because Ostreopsis exhibits both benthic and planktonic phases (Bravo et al., Reference Bravo, Vila, Casabianca, Rodríguez, Rial, Riobó and Penna2012), its dispersion is facilitated, increasing the number of seafood types that could become contaminated and complicating its monitoring. The planktonic stage can contaminate filter-feeding bivalves (e.g. mussels), while the benthic stage can enter the food chain through herbivores (e.g. sea urchins, crabs, gastropods, salps) that feed on macroalgae (Brissard et al., Reference Brissard, Herrenknecht, Séchet, Hervé, Pisapia, Harcouet, Lémée, Chomérat, Hess and Amzil2014). PLTX-like biotoxins can then be transferred to omnivores and carnivores, including humans.
EMERGING BIOTOXINS
Cyclic imines
Cyclic imines (gymnodimine, spirolides, pinnatoxins and others) have been produced reportedly by a number of dinoflagellates (Karenia selliformis, Vulcanodinium rugosum, Alexandrium ostenfeldii and A. peruvianum). They are classified typically as fast-acting biotoxins due to the rapid mortality occurring in the mouse bioassay for lipophilic biotoxins (Molgó et al., Reference Molgó, Aráoz, Benoit, Iorga and Botana2014, and references cited therein). Both gymnodimine and pinnatoxins had been associated initially with acute human food poisoning events (Seki et al., Reference Seki, Satake, MacKenzie, Kaspar and Yasumoto1995; Uemura et al., Reference Uemura, Chou, Haino, Nagatsu, Fukuzawa, Zheng and Chen1995). Further studies indicated that other biotoxins (i.e. brevetoxins in the case of the New Zealand shellfish poisoning assumed to be caused by gymnodimine) or bacterial (Vibrio) contamination (in the case of the Chinese poisoning assumed to be caused by pinnatoxins) were in fact responsible. Pinnatoxin-G has been reported worldwide (e.g. Rhodes et al., Reference Rhodes, Smith, Selwood, McNabb, van Ginkel, Holland and Munday2010, Reference Rhodes, Smith, Selwood, McNabb, Munday, Suda, Molenaar and Hallegraeff2011; Rundberget et al., Reference Rundberget, Aasen, Selwood and Miles2011; McCarron et al., Reference McCarron, Rourke, Hardstaff, Pooley and Quilliam2012), with the highest levels found in a Mediterranean lagoon in the south of France (Hess et al., Reference Hess, Abadie, Herve, Berteaux, Sechet, Araoz, Molgo, Zakarian, Sibat, Rundberget, Miles and Amzil2013). It should be pointed out, however, that no food-poisoning-related events have yet been documented. The chemical stability of this biotoxin to both acids and bases (Jackson et al., Reference Jackson, Stivala, Iorga, Molgo and Zakarian2012) and the high binding affinity to the nicotinic acetylcholine (nACh) receptor raises the spectre of potentially harmful effects to human health due to the chronic exposure of even low levels of pinnatoxins (Molgó et al., Reference Molgó, Aráoz, Benoit, Iorga and Botana2014).
BMAA
β-N-methylamino-L-alanine (BMAA), a non-protein amino acid initially reported to be produced by certain cyanobacteria proliferating in freshwater habitats (Cox et al., Reference Cox, Banack, Murch, Rasmussen, Tien, Bidigare, Metcalf, Morrison, Codd and Bergman2005), has been recently confirmed in marine diatoms (Jiang et al., Reference Jiang, Eriksson, Lage, Jonasson, Shams, Mehine, Ilag and Rasmussen2014a) and in seafood from marine and coastal waters (Brand et al., Reference Brand, Pablo, Compton, Hammerschlag and Mash2010; Réveillon et al., Reference Réveillon, Abadie, Sechet, Brien, Savar, Bardouil, Hess and Amzil2014, Reference Réveillon, Abadie, Séchet, Masseret, Hess and Amzil2015). Exposure to BMAA has been linked to an increased risk of neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), Parkinson's and Alzheimer's diseases (Bradley et al., Reference Bradley, Borenstein, Nelson, Codd, Rosen, Stommel and Cox2013). Marine cyanotoxins in general are a potential cause of unexplained acute food poisoning (Golubic et al., Reference Golubic, Abed, Palińska, Pauillac, Chinain and Laurent2010; Roué et al., Reference Roué, Cruchet, Ung, Darius, Chinain and Laurent2013, Reference Roué, Gugger, Golubic, Amzil, Aráoz, Turquet, Chinain, Laurent, La Barre and Kornprobst2014). Additional research is needed to confirm both these findings and the possible epidemiological associations between BMAA and neurodegenerative diseases.
Macro-algae
Food poisoning from macroalgae has been reported from Asian countries where such foodstuffs are traditionally consumed. Particularly important are the polycavernosides found in Gracilaria edulis (Louzao et al., Reference Louzao, Vilariño, Yotsu-Yamashita and Botana2014, and references cited therein). As postulated by Daigo (Reference Daigo1959), domoic acid has now been confirmed as a metabolite in Chondria armata following the laboratory culture of these macroalgae (Jiang et al., Reference Jiang, Kuwano, Ishikawa, Yano, Takatani and Arakawa2014b). The occurrence of domoic acid and other glutamate receptor agonists in macroalgae may be of importance in studies on the effects of chronic exposures to subacute biotoxin concentration.
EFFECTS OF CHRONIC AND SUBACUTE EXPOSURE
Marine biotoxins have traditionally been known for their acute effects. Consequently, regulation at a global scale (FAO, Codex alimentarius) has focused on preventing acute poisoning events (Lawrence et al., Reference Lawrence, Loreal, Toyufuku, Hess, Karunasagar and Ababouch2011). Only a few studies have investigated either chronic effects from an acute exposure episode or chronic exposure to sub-acute levels over time in humans. Some studies, using in vivo models, have shown that there may be some adverse health effects from low-level exposures (either single or repeated) to, for example, domoic acid. Levin et al. (Reference Levin, Pizarro, Pang, Harrison and Ramsdell2005) demonstrated that prenatal rats exposed to domoic acid exhibited postnatal effects, e.g. scopolamine susceptibility. In this study, rats showed persistent hypo-activity, and female offspring in particular showed poor performance in a maze, following a single low-dose early postnatal exposure to domoic acid. Baron et al. (Reference Baron, Steeg, Morris, Blain and Judge2011) reported locomotor disorders in rats exposed to a low level of domoic acid. Finally, low-level repeated exposure over a 36-week period in zebra fish also led to gene alteration and impairment of mitochondrial function at cellular level (Lefebvre et al., Reference Lefebvre, Frame, Gulland, Hansen, Kendrick, Beyer, Bammler, Farin, Hiolski, Smith and Marcinek2012; Hiolski et al., Reference Hiolski, Preston, Frame, Myers, Bammler, Beyer, Farin, Wilkerson, Smith, Marcinek and Lefebvre2014).
Sub-acute effects are also a potential problem for repeated or chronic ingestion of low doses of saxitoxins via drinking water produced from desalination of seawater. Until now, saxitoxins have been regulated only on the basis of their acute effects: a maximum permissible level of 3 µg L−1 has been established for drinking water in Australia, Brazil and New Zealand. Recent studies suggest, however, that the effects of chronic ingestion of saxitoxins may include the alteration of antioxidant defences and the induction of oxidative stress in the brains and livers of mammals (Ramos et al., Reference Ramos, Dieh, dos Santos, Monserrat and Yunes2014; Silva et al., Reference Silva, Morais, Costa, Ribas, Guiloski, Ramsdorf, Zanata, Cestari, Oliveira Ribeiro, Magalhaes, Trudeau and Silva de Assis2014). As mentioned above, the cyclic imine biotoxins also have the potential to interact with receptors involved in neurodegenerative diseases such as Parkinson's and Alzheimer's diseases (Molgó et al., Reference Molgó, Aráoz, Benoit, Iorga and Botana2014). Interestingly, in both in vitro and in vivo studies, gymnodimine and 13-desmethyl spirolide C showed some protective effects against Alzheimer's disease, and hence these compounds should be considered for further research into their possible therapeutic applications (Alonso et al., Reference Alonso, Vale, Vieytes, Laferla, Gimenez-Llort and Botana2011a, Reference Alonso, Vale, Vieytes, Laferla, Gimenez-Llort and Botanab, Reference Alonso, Otero, Vale, Alfonso, Antelo, Giménez-Llort, Chabaud, Guillou and Botana2013).
CO-EXPOSURE AND EXPOSURE TO MIXTURES
Co-exposure to different agents can lead to confounding diagnoses in patients. For example, bacterial contamination in shellfish may lead to gastrointestinal illness (diarrhoea and vomiting) similar in degree to that caused by okadaic acid. Normally, the onset of illness following the consumption of shellfish contaminated with biotoxins is earlier (a few hours) than for those contaminated with bacterial pathogens (12–24 h), because bacteria typically require a period of incubation in the patient before causing the full effect.
Exposure to mixtures of different groups of biotoxins is another issue that has not been well studied. Many studies have reported the co-occurrence of biotoxins in single algal species or shellfish that were contaminated by different algal species simultaneously, so the issue should be considered seriously (Hess, Reference Hess, Villalba, Reguera, Romalde and Beiras2002, Reference Hess2010; Amzil et al., Reference Amzil, Sibat, Royer and Savar2008; Twiner et al., Reference Twiner, Rehmann, Hess and Doucette2008; Reguera et al., Reference Reguera, Velo-Suárez, Raine and Park2012; Suikkanen et al., Reference Suikkanen, Kremp, Hautala and Krock2013). In particular, there is a concern that some of the compounds that do not normally cause acute shellfish poisoning in humans (e.g. yessotoxins), may be absorbed following damage to the intestinal tract caused by other biotoxins known to cause harm to humans, (e.g. okadaic acid and azaspiracids). As pure biotoxin supplies are very limited for most of the HAB biotoxins, only a few studies have investigated this potential hazard. Aasen et al. (Reference Aasen, Espenes, Miles, Samdal, Hess and Aune2011) investigated the combination of azaspiracids with yessotoxins and did not observe any potentiation when orally co-administered at sub-acute levels to mice. Similarly, even a combination of azaspiracids with okadaic acid, two biotoxins known for their potential to cause harm to the human digestive tract, did not cause synergistic effects when orally administered to mice at sub-acute levels (Aune et al., Reference Aune, Espenes, Aasen, Quilliam, Hess and Larsen2012). The combination of okadaic acid and yessotoxins did not cause any increased toxicity in the oral mouse model (Sosa et al., Reference Sosa, Ardizzone, Beltramo, Vita, Dell'Ovo, Barreras, Yasumoto and Tubaro2013). It should be noted that the digestive tract of mice differs significantly from that of humans (notably in pH), however, and further studies (possibly involving primates) may be necessary to rule out the likelihood of synergistic damage. Finally, some potentiation of domoic acid by peptaibols, a fungal metabolite, was shown in a fly larval model (Ruiz et al., Reference Ruiz, Petit, Vansteelandt, Kerzaon, Baudet, Amzil, Biard, Grovel and Pouchus2010). These varied findings are very much dependent on the biological model used, and they should be considered as very preliminary in their nature. Longitudinal studies of humans exposed naturally to mixtures of biotoxins at individually low levels over long periods of time are needed.
Water-borne diseases
In fresh waters, cyanobacteria blooms constitute the main hazard to the health of humans (and other animals), mainly through the contamination of drinking waters by cyanotoxins, such as microcystins (see e.g. Nishiwaki-Matsushima et al., Reference Nishiwaki-Matsushima, Ohta, Nishiwaki, Suganuma, Kohyama, Ishikawa, Carmichael and Fujiki1992; Falconer, Reference Falconer and Hrub1998; Stewart et al., Reference Stewart, Seawright and Shaw2008). Direct cutaneous contact, exposure to aerosols, or swallowing water during occupational or recreational activities conducted in freshwater environments affected by cyanobacteria blooms, also can cause health problems. In contrast, there is no published evidence, at present, of risks for adverse health effects in humans from swallowing seawater containing toxic marine microalgae, but this possibility cannot be discarded.
Toxic HABs have recently emerged as a potential risk for the contamination of drinking water supplied by desalination systems. Worldwide, desalination is rapidly growing to provide water for domestic consumption and industrial uses. In 2012, there were more than 14,000 desalination plants in 150 countries (Anderson & McCarthy, Reference Anderson and McCarthy2012). About 50% of this capacity was located in the West Asia Gulf region, 17% in North America, 10% in Asia (apart from the Gulf), 8% in North Africa and 7% in Europe. In 2008, the installed capacity was 52.3 million m3 per day. Based on a growth rate of 12% per year, the global production of freshwater by desalination will have reached a capacity of 94 million m3 per day by 2015.
Chemical and physical properties, such as the molecular weight of the common HAB biotoxins (saxitoxins, brevetoxins and domoic acid, i.e. 300–900 Da), suggest that they should be efficiently removed by reverse osmosis in the desalinization process. Support for this assumption was provided by Seubert et al. (Reference Seubert, Trussell, Eagleton, Schnetzer, Cetinic, Lauri, Jones and Caron2012) in their study combining laboratory tests and a 5-year monitoring of an operational plant in California. It has been noticed, however, that some taste and odour compounds (e.g. geosmin) with low molecular weights similar to the biotoxins mentioned above can pass through pre-treatment and reverse osmosis membranes (Reiss et al., Reference Reiss, Robert, Owen and Taylor2006). Further, the complete removal of biotoxins is not guaranteed in membranes with micro-fissures caused by, for instance, high pressures within the desalination plant system. Excessive pressures can be caused by the obstruction of intake filters due to high-biomass blooms, such as the ones caused by Cochlodinium polykrikoides that occurred in the Arabian Gulf and Gulf of Oman (Richlen et al., Reference Richlen, Morton, Jamali, Rajan and Anderson2010). Still, the data are limited (e.g. Caron et al., Reference Caron, Gurnee, Seubert, Howard, Darjany, Schnetzer, Cetinic, Filteau, Lauri, Jones and Trussell2010; Dixon et al., Reference Dixon, Falconet, Ho, Chow, O'Neill and Newcombe2011a, Reference Dixon, Richard, Ho, Chow, O'Neill and Newcombeb; Laycock et al., Reference Laycock, Anderson, Naar, Goodman, Easy, Donovan, Li, Quilliam, Al Jamali and Alshihi2012), based mainly on laboratory studies (without the appropriate up-scaling), and there are only a few studies (Seubert et al., Reference Seubert, Trussell, Eagleton, Schnetzer, Cetinic, Lauri, Jones and Caron2012) from plants operating during blooms of biotoxin-producing HAB species. Therefore, more research is needed to ascertain the fate of biotoxins during the desalination process. The potential risk of chronic exposure to biotoxins requires their monitoring in drinking water produced in desalination plants.
HABs and aerosolized biotoxins
KARENIA BREVIS AND BREVETOXINS
Over three decades, multi-institutional and multidisciplinary studies in the Gulf of Mexico and along the coast of Florida have explored how the inhalation of aerosols containing brevetoxins during high-biomass, toxic Karenia brevis blooms can cause respiratory symptoms (e.g. Fleming et al., Reference Fleming, Kirkpatrick, Backer, Bean, Wanner, Dalpra, Tamer, Zaias, Cheng, Pierces, Naar, Abraham, Clark, Zhou, Henry, Johnson, Van de Bogart, Bossart, Harrington and Baden2005, Reference Fleming, Kirkpatrick, Backer, Bean, Wanner, Reich, Zaias, Cheng, Pierce, Naar, Abraham and Baden2006, Reference Fleming, Kirkpatrick, Backer, Walsh, Nierenberg, Clark, Reich, Hollenbeck, Benson, Cheng, Naar, Pierce, Bourdelais, Abraham, Kirkpatrick, Zaias, Wanner, Mendes, Shalat, Hoagland, Stephan, Bean, Watkins, Clarke, Byrne and Baden2011). Beach visitors and full-time lifeguards have reported respiratory disorders (Backer et al., Reference Backer, Fleming, Rowan, Cheng, Benson, Pierce, Zaias, Bean, Bossart, Johnson, Quimbo and Baden2003, Reference Backer, Kirkpatrick, Fleming, Cheng, Pierce, Bean, Clark, Johnson, Wanner, Tamer and Baden2005), and studies have shown that people with asthma (and possibly other lung diseases) were particularly at risk from more severe and longer-lasting symptoms (Fleming et al., Reference Fleming, Kirkpatrick, Backer, Bean, Wanner, Dalpra, Tamer, Zaias, Cheng, Pierces, Naar, Abraham, Clark, Zhou, Henry, Johnson, Van de Bogart, Bossart, Harrington and Baden2005, Reference Fleming, Kirkpatrick, Backer, Bean, Wanner, Reich, Zaias, Cheng, Pierce, Naar, Abraham and Baden2006, Reference Fleming, Kirkpatrick, Backer, Walsh, Nierenberg, Clark, Reich, Hollenbeck, Benson, Cheng, Naar, Pierce, Bourdelais, Abraham, Kirkpatrick, Zaias, Wanner, Mendes, Shalat, Hoagland, Stephan, Bean, Watkins, Clarke, Byrne and Baden2011; Milian et al., Reference Milian, Nierenberg, Fleming, Bean, Wanner, Reich, Backer, Jayroe and Kirkpatrick2007; Bean et al., Reference Bean, Fleming, Kirkpatrick, Backer, Nierenberg, Reich, Cheng, Wanner, Benson, Naar, Pierce, Abraham, Kirkpatrick, Hollenbeck, Zaias, Mendes and Baden2011). More recent research has modelled the health costs of these effects (Hoagland et al., Reference Hoagland, Jin, Polansky, Kirkpatrick, Kirkpatrick, Fleming, Reich, Watkins, Ullmann and Backer2009, Reference Hoagland, Jin, Beet, Kirkpatrick, Reich, Ullman, Fleming and Kirkpatrick2014).
The results of this research have been used to engage with stakeholders to develop local response management plans to help minimize societal impacts of Karenia blooms on human health (e.g. Kirkpatrick et al., Reference Kirkpatrick, Bean, Fleming, Kirkpatrick, Grief, Nierenberg, Reich, Watkins and Naar2010; Fleming et al., Reference Fleming, Kirkpatrick, Backer, Walsh, Nierenberg, Clark, Reich, Hollenbeck, Benson, Cheng, Naar, Pierce, Bourdelais, Abraham, Kirkpatrick, Zaias, Wanner, Mendes, Shalat, Hoagland, Stephan, Bean, Watkins, Clarke, Byrne and Baden2011; Zhao et al., Reference Zhao, Hu, Lener, Weisberg, Lembke, English, Wolny, Zheng, Walsh and Kirkpatrick2013). Information about the likelihood of health impacts has been used to inform personal decisions as well. For example, the Gulf of Mexico HAB Forecast (see NOAA Harmful Algal Bloom Operational Forecast System (HAB-OFS), https://tidesandcurrents.noaa.gov/hab/, accessed 26 August 2015) in conjunction with a local Beach Condition Reporting System is used by windsurfers and beach visitors to decide, depending on the wind, tides and the presence of a Florida red tide, whether or not to visit a particular beach on a given day. Thus, current observations and forecasts of this HAB may help to protect public health by decreasing exposures to toxic aerosols.
OSTREOPSIS CF. OVATA AND RESPIRATORY IRRITATIONS
Over the past two decades, extensive blooms of Ostreopsis spp. have occurred in the Mediterranean (e.g. Algeria, France, Italy, Tunisia). During these blooms, beach users have experienced upper respiratory disorders (rhinorrhoea), eye and nose irritation, fever and general malaise, and sought medical care in hospital emergency departments and primary healthcare centres (Àlvarez et al., Reference Àlvarez, Pedrol, Parrón, Goxencia, de Torres, Manzanera, Planas, Cosculluela and López2005; Gallitelli et al., Reference Gallitelli, Ungaro, Addante, Silver and Sabbà2005; Brescianini et al., Reference Brescianini, Grillo, Melchiorre, Bertolotto, Ferrari, Vivaldi, Icardi, Gramaccioni, Funari and Scardala2006; Durando et al., Reference Durando, Ansaldi, Oreste, Moscatelli, Marensi, Grillo, Gasparini and Icardi2007; Barroso et al., Reference Barroso García, Rueda de la Puerta, Parron Carreno, Marín Martínez and Enriquez2008; Vila et al., Reference Vila, Masó, Sampedro, Illoul, Arin, Garcés, Giacobbe, Àlvarez, Camp, Moestrup, Doucette, Enevoldsen, Godhe, Hallegraeff, Luckas, Lundholm, Lewis, Rengefors, Selner, Steidinger, Tester and Zingone2008, Reference Vila, Arin, Battocchi, Bravo, Fraga, Penna, Reñé, Riobó, Rodríguez, Sala, Camp, de Torres and Franco2012; Tichadou et al., Reference Tichadou, Glaizal, Armengaud, Grossel, Lemée and Kantin2010; Tubaro et al., Reference Tubaro, Durando, Del Favero, Ansaldi, Icardi and Deeds2011; Illoul et al., Reference Illoul, Rodríguez, Vila, Adjas, Aït Younes, Bournissa, Koroghli, Marouf, Rabia and Ameur2012). A similar situation could also be occurring in certain beaches in Brazil (Proença et al., Reference Proença, Boemer, Dias, Hatherly, Mendes, Mendes, Mendes, Rossi, Tamanaha, Tenenbaum and Schramm2010). The health effects could be caused by exposures to aerosols containing biotoxins or allergenic substances produced by Ostreopsis cell fragments or accompanying microbiota (Casabianca et al., Reference Casabianca, Casabianca, Riobó, Franco, Vila and Penna2013; Ciminiello et al., Reference Ciminiello, Dell'Aversano, Dello Iacovo, Fattorusso, Forino and Tartaglione2014). Reported cases of cutaneous irritation were attributed (but not proven) to direct skin contact with seawater containing high concentrations of Ostreopsis cells. At present, studies (including epidemiological and ecological) are underway to improve the understanding of Ostreopsis bloom dynamics and the possible links to human health effects (Vila et al., Reference Vila, Riobó, Bravo, Masó, Penna, Reñé, Pagou and Hallegraeff2014; Berdalet et al., Reference Berdalet, Vila and Abós-Herràndiz2015).
EFFECTS OF HABS ON HUMAN WELLBEING
In addition to the risks to human health posed by exposure to biotoxins, marine HABs can impact other aspects of human wellbeing, including human commercial and recreational uses of the coastal and marine environments, such as fishing, shellfish collection and growing, and tourism, and non-market, passive uses of the ocean, such as preferences for particular ecological states. Also, marine HABs may lead to complex societal responses in the affected human communities, such as the more general avoidance of coastal and ocean resources due to misconceptions and inadequate communications of health risks. In this section, we refer to the economic effects resulting both from biotoxin producing species (Box 1) and from algal taxa involved in high biomass events (Box 2). Difficulties in developing estimates of the economic costs associated with HABs are considered in detail in this section.
Box 2. Representative examples of HABs that affect marine organisms or ecosystems, with effects on wellbeing, and on human health in some cases.
Events presented following the alphabetical order of the Causative organism (CO). The Type (T) of bloom, their Effect/mode of action in the marine organisms or ecosystem (E/m-a), Effects on humans (E-h), Main Geographic Affected Areas (A) and some References (R) are indicated.
General information. Certain HABs affect marine organisms and ecosystems, due to the accumulation of biomass of particular taxa, which may or may not produce biotoxins In most cases, fish-killing HAB species produce haemolytic or cytotoxic biotoxins, which cause necrotizing degeneration of the fish gills. Other, more rare or more recently discovered combinations of fish-killing algae and their biotoxins include goniodomins (produced by G. pseudogonyaulax), amphidinols (synthesized by Amphidinium klebsii and A. carteri; reviewed by Louzao et al., Reference Louzao, Vilariño, Yotsu-Yamashita and Botana2014) and karlotoxins from Karlodinium spp. (Bachvaroff et al., Reference Bachvaroff, Adolf, Squier, Harvey and Place2008; Place et al., Reference Place, Munday and Munday2014; Van Wagoner et al., Reference Van Wagoner, Deeds, Tatters, Place, Tomas and Wright2010). In many cases though, the toxic compound has not yet been clearly identified.
The breakdown of cells during the decline of a bloom, with the subsequent degradation and utilization of the organic matter by bacteria can result in low oxygen concentrations of isolated bottom water, causing subsequent macrofauna mortalities. Other impacts on the ecosystem include: the reduction of light penetration, production of excess ammonia, physical damage of fish gills, and hypothermia in marine birds due to the accumulation of surfactant-like proteins Here we list examples of many microalgae and a macrophyte involved in HAB events. More details and examples of these blooms, mainly high biomass blooms, can be found, among others, in e.g. GEOHAB, Reference Glibert and Pitcher2001, Reference Glibert2006, Reference Furuya, Glibert, Zhou and Raine2010 (download free from http://www.geohab.info).
CO: Akashiwo sanguinea (Dinophyceae)
T: High biomass, non-toxic
E/m-a: Extensive marine birds mortality caused by hypothermia. The microalgae produced high amounts of organic matter that accumulated at the sea surface. The foam contained surfactant-like proteins, which destroyed the waterproof and insulation characteristics of the bird feathers
E-h: Unknown
R: Jessup et al. (Reference Jessup, Miller, Ryan, Nevins, Kerkering, Mekebri, Crane, Johnson and Kudela2009)
A: Pacific North-west (Monterey Bay, California)
CO: Chaetoceros wighami, C. debile (Bacillariophyceae); Dictyocha speculum (Silicoflagellate) T: High biomass, non-toxic. Physical damage (silicic cell cover) of the fills gills E/m-a: Farmed fish kills
E-h: Economic losses (several million pounds)
R: Bruno et al. (Reference Bruno, Dear and Seaton1989); Treasurer et al. (Reference Treasurer, Hannah and Cox2003)
A: Europe (Loch Torridon, Scotland; Shetland Isles)
CO: Chattonella antiqua and C. marina (Raphidophyceae)
T: High biomass, toxic (haemolytic, haemagglutinating and neurotoxic effects, molecule similar to brevetoxin)
E/m-a: Aquaculture fish and natural fauna kills
E-h: Economic losses (US$0.5 billion in 1972; Australia AU$45.0 million losss of caged bluefin tuna in April 1996)
R: Endo et al. (Reference Endo, Sakai and Kuroki1985), Marshall & Hallegraeff (Reference Marshall and Hallegraeff1999), and references cited therein
A: Japan, Australia, India, Florida and China
CO: Cochlodinium polykrikoides, Cochlodinium spp. (Dinophyceae)
T: High biomass, toxic. The toxic compound and mechanism have not been identified yet, with controversial results (Tang & Gobler, Reference Tang and Gobler2008, and refs therein). Toxicity could be caused by non-hydrogen peroxide, highly reactive, labile biotoxins such as ROS-like chemicals
E/m-a: Wild and farmed fish kills; coral and shellfish
E-h: Economic losses. Hundreds of millions of US$ in fisheries losses in Korea alone; more than US$3.0 million in Vancouver (in 1999); in the Arabian Gulf and Gulf of Oman, the long-lasting Cochlodinium bloom of 2010 killed thousands of tonnes of fish, limited traditional fishery operations, damaged coral reefs, impacting coastal tourism, and forcing the closure of desalination systems
R: Yuki & Yoshimatsu (Reference Yuki, Yoshimatsu, Okaichi, Anderson and Nemoto1989); Kim et al. (Reference Kim, Lee, Lee, Kim and Jung1999); Whyte et al. (Reference Whyte, Haigh, Ginther and Keddy2001); Gobler et al. (Reference Gobler, Berry, Anderson, Burson, Koch, Rodgers, Moore, Goleski, Allam, Bowser, Tang and Nuzzi2008); Richlen et al. (Reference Richlen, Morton, Jamali, Rajan and Anderson2010); Kudela & Gobler (Reference Kudela and Gobler2012), and references cited therein
A: North America, Asia, Australia and Europe
CO: Heterosigma akashiwo (Raphidophyceae)
T: High biomass, toxic. No identified toxic nor toxicity mechanism, which may affect other aquatic organisms (zooplankton, copepods, benthic larvae)
E/m-a: Aquaculture fish kills
E-h: Economic losses
R: Black et al. (Reference Black, Whyte, Bagshaw and Ginther1991), Yamochi (Reference Yamochi, Okaichi, Anderson and Nemoto1989); MacKenzie (Reference MacKenzie1991); Powers et al. (Reference Powers, Creed and Trick2015)
A: Atlantic and Pacific coast: Canada, Chile, Japan and New Zealand
CO: Karenia brevis (Dinophyceae)
T: High biomass, toxic (brevetoxin, haemolytic)
E/m-a: Toxicity to humans and marine fauna by direct ingestion or aerosolization; manatee mortality during the active bloom phase; dolphin and manatee poisoning and mortality through the ingestion of contaminated fish and aquatic plants during the senescence phase of the bloom. Fish kills
E-h: Health costs; tourism losses, passive use losses; costs of beach cleanups
R: Tangen (Reference Tangen1977); Steidinger et al. (Reference Steidinger, Vargo, Tester, Tomas, Anderson, Cembella and Hallegraeff1998); Bossart et al. (Reference Bossart, Baden, Ewing, Wright and Pfeiffer2002); Magaña et al. (Reference Magaña, Contreras and Villareal2003); Flewelling et al. (Reference Flewelling, Naar, Abbott, Baden, Barros, Bossart, Bottein, Hammond, Haubold, Heil, Henry, Jacocks, Leighfield, Pierce, Pitchford, Rommel, Scott, Steidinger, Truby, Van Dolah and Landsberg2005); Naar et al. (Reference Naar, Flewelling, Lenzi, Abbott, Granholm, Jacocks, Gannon, Henry, Pierce, Baden, Wolny and Landsberg2007); Campbell et al. (Reference Campbell, Henrichs, Olson and Sosik2013)
A: Gulf of Mexico, Florida, North Carolina
CO: Karenia mikimotoi (Dinophyceae)
T: High biomass, toxic (gimnocin, haemolytic)
E/m-a: Fish kills. Mortalities of marine fauna
E-h: Economic losses; passive use losses
R: Dahl & Tangen (Reference Dahl, Tangen, Granéli, Sundstrøm, Edler and Anderson1990, Reference Dahl, Tangen, Smayda and Shimizu1993); Nakamura et al. (Reference Nakamura, Suzuki and Hiromi1995); Gentien (Reference Gentien, Anderson, Cembella and Hallegraeff1998); Raine et al. (Reference Raine, O'Boyle, O'Higgins, White, Patching, Cahill and McMahon2001); Satake et al. (Reference Satake, Shoji, Oshima, Naoki, Fujita and Yasumoto2002); Silke et al. (Reference Silke, O'Beirn and Cronin2005); Vanhoutte et al. (Reference Vanhoutte-Brunier, Fernand, Ménesguen, Lyons, Gohin and Cugier2008); Davidson et al. (Reference Davidson, Miller, Wilding, Shutler, Bresnan, Kennington and Swan2009)
A: Asian and Australian, Northern European waters
CO: Noctiluca (heterotrophic Dinophyceae)
T: High biomass, non-toxic
E/m-a: Hypoxia and excess ammonia in the environment; unpleasant odours; water discolouration
E-h: Economic losses (on tourism, not estimated)
R: Bricelj & Lonsdale (Reference Bricelj and Lonsdale1997); Elbrächter & Qi (Reference Elbrächter, Qi, Anderson, Cembella and Hallegraeff1998); Murray & Suthers (Reference Murray and Suthers1999); Fonda-Umani et al. (Reference Fonda-Umani, Beran, Parlato, Virgilio, Zollet, De Olazabal, Lazzarini and Cabrini2004)
A: Worldwide
CO: Ostreopsis cf. ovata, O. siamensis (benthic Dinophyceae)
T: High biomass, toxic
E/m-a: Cells attach to corals, macrophytes or macrofauna by mucous substances. Toxicity (Palytoxin and analogues) to marine fauna by direct ingestion or by anoxia. The particular mechanisms have not been identified yet
E-h: Respiratory irritation, possible gastrointestinal illness
R: Shears & Ross (Reference Shears and Ross2009); Mangialajo et al. (Reference Mangialajo, Ganzin, Accoroni, Asnaghi, Blanfune, Cabrini, Cattaneo-Vietti, Chavanon, Chiantore, Cohu, Costa, Fornasaro, Grossel, Marco-Miralles, Masó, Reñé, Rossi, Sala, Thibaut, Totti, Vila and Lemée2011) and references cited therein
A: Mediterranean, New Zealand, Brazil, Japan
CO: Phaeocystis (Prymnesiophyceae)
T: High biomass, non-toxic
E/m-a: Production of foam or mucilage (alteration of gas diffusion and rheologic properties) causing farmed fish kills; discolouration and repellent odour can impact tourism
E-h: From ~US$0.7 million in Vietnam to ~US$7.5 million in Asian aquaculture (1997, Quanzhan Bay, Fujian province, China)
R: Lancelot et al. (Reference Lancelot, Billen, Sournia, Weisse, Colijn, Veldhuis, Davies and Wassman1987); Schoemann et al. (Reference Schoemann, Becquevort, Stefels, Rousseau and Lancelot2005); Hai et al. (Reference Hai, Lam and Dippner2010) and references cited therein; Arin et al. (Reference Arin, Guillén, Segura-Noguera and Estrada2013)
A: Asian coastlines, North Sea, Antarctica
CO: Prymnesium parvum (Prymnesiophyte)
T: High biomass, toxic. A variety of toxic compounds with lytic effects (allelopathy) that would affect other organisms in the food webs, as well as haemolytic and cytotoxic effects on fishes (Blossom et al., Reference Blossom, Andersen, Rasmusse and Hansen2014)
E/m-a: Aquaculture fish kills
E-h: Economic losses (US$5.0 million)
R: Kaartvedt et al. (Reference Kaartvedt, Johnsen, Aksens, Lie and Svendsen1991)
A: Norwegian fjords
CO: Pseudochattonella verruculosa (Dictyochophyceae)
T: High biomass, toxic
E/m-a: Aquaculture fish kills
E-h: Economic losses
R: Mackenzie et al. (Reference MacKenzie, Smith, Rhodes, Brown, Langi, Edgar, Lovell and Preece2011)
A: New Zealand
CO: Pseudo-nitzschia spp. (Bacillariophyceae)
T: High biomass, toxic (domoic acid, neurotoxic)
E/m-a: Morbidity and mortality of large vertebrates, including seabirds, sea lions, sea otters, selas, and possibly whales
E-h: Possible passive use losses
R: Sierra-Beltrán et al. (Reference Sierra-Beltrán, Palafox-Uribe, Grajales-Montiel, Cruz-Villacorta and Ochoa1997); Scholin et al. (Reference Scholin, Gulland, Doucette, Benson, Busman, Chavez, Cordaro, DeLong, De Vogelaere, Harvey, Haulena, Lefebvre, Lipscomb and Van Dolah2000); Hall & Frame (Reference Hall and Frame2010)
A: Especially, Pacific coast of North America
CO: Ulva (Ulvaceae), macroscopic benthic macroalgae
T: High biomass, non toxic
E/m-a: Hypoxia in the environment; unpleasant odours; impacts on tourism
E-h: Beach cleanup costs: US$87.3 million in China; US$10.3–165.9 million in France, depending on the extent of affected areas (approximate period 1989–2006)
R: Hu & He (Reference Hu and He2008); Zhou et al. Reference Zhou, Liu, Anderson and Valiela2015 and references cited therein.
A: China (Qingdao) during the Olympics, Atlantic French coast
In China, disrupted activities during the Olympic games in 2008
Economic effects of HABs linked to food security and seafood-related activities
Certain HABs constitute a threat to aquaculture food production, which has become a major source of protein (linked to decreasing wild fish stocks) for expanding human populations, particularly in coastal communities in developing countries. Like farming, and sometimes in conjunction with it, aquaculture helps to support many local and regional economies (FAO, 2006). Currently, and with respect to the cultivation (and wild-harvest) of shellfish, the most effective way to protect humans from HAB-related seafood poisoning is to monitor for the presence of HAB species or biotoxins and to enforce periodic closures of commercial and recreational harvesting or growing areas (Figure 3). Contamination of seafood products can result in economic losses in shellfish collection and growing, and in the aquaculture of certain finfish. There may also be losses in ancillary industries, including processing, distribution, wholesaling and retailing of seafood (Larkin & Adams, Reference Larkin and Adams2007; Morgan et al., Reference Morgan, Larkin and Adams2010).

Fig. 3. Main elements of the international GEOHAB (2000–2014) and GlobalHAB (2015–2018) programmes, that aim to contribute to the advance of the research on HABs.
Estimating the overall economic costs associated with the occurrence of HABs is complex. These costs vary markedly, depending on geographic region, seafood product, the frequency and intensity of HAB-related events, the duration of fishery closures and the costs of healthcare provision. As an example, Hoagland et al. (Reference Hoagland, Anderson, Kaoru and White2002) estimated the annual economic effects of HABs in the USA between 1987 and 1992. At that time, the economic effects were valued in the order of US$50.0 million per year; this calculation has been subsequently adjusted for inflation to ~US$100.0 million per year (Hoagland & Scatasta, Reference Hoagland, Scatasta, Granéli and Turner2006). It is important to note that there is considerable variation in estimated impacts from year to year. This estimate was based on a compilation of the assessments of economic effects in public health, commercial fisheries, recreation and tourism, and monitoring and management. Notably, public health effects are the largest component, representing about 42% of nationwide average effects during that time period. Although the actual incidence of ciguatera fish poisoning (CFP) is very uncertain in many tropical locations, estimates of CFP illnesses comprise 99% of the total public health costs.
With regards to the specific economic costs of HAB-induced illnesses, there are very few studies available. The UNEP Global Environmental Outlook lists the worldwide economic impacts of algal biotoxins on human health from seafood alone as ~US$4.0 billion a year (GESAMP, 2001). As a comparison, the impact of infectious hepatitis from seafood consumption was estimated to be ~US$7.2 billion per year. The direct medical care costs associated with the respiratory and digestive illnesses caused by K. brevis blooms along the Florida coast of the Gulf of Mexico ranged from US$0.1–0.7 million annually, depending on the intensity of the event (Hoagland et al., Reference Hoagland, Jin, Polansky, Kirkpatrick, Kirkpatrick, Fleming, Reich, Watkins, Ullmann and Backer2009, Reference Hoagland, Jin, Beet, Kirkpatrick, Reich, Ullman, Fleming and Kirkpatrick2014). Illness costs might exceed US$1.0 million per year for large, long-lasting blooms, however, such as one that occurred during 2005–06. Assuming that the average annual illness costs of K. brevis blooms would persist into the future, using a discount rate of 3%, the capitalized costs of future illnesses would range between US$2.0–24.0 million.
In the particular case of ciguatera fish poisoning, the average medical costs of diagnosis and treatment in traditionally non-endemic areas, as for instance in Canada, was estimated at about CA$2470 per case, with about 1000 cases per year related to tourism and food importation in 1990 (Lange et al., Reference Lange, Snyder and Fudala1992; Todd, Reference Todd, Lassus, Arzul, Erard, Gentien and Marcaillou-Le Bout1995; Fleming et al., Reference Fleming, Backer, Rowan, Baden and Adams2002). Accurate estimates of the human costs of these diseases necessitate an adequate knowledge of their prevalence and incidence, as well as an understanding of their acute and chronic human health effects (Pennotti et al., Reference Pennotti, Scallan, Backer, Thomas and Angulo2013).
Any estimation of the economic effects of HABs also should include an evaluation of the costs of monitoring and management, which would include, for instance, sampling programmes or strategies to decrease the risk of HAB occurrences (e.g. reducing coastal eutrophication, sustainable use of the coastal zone, and other human-related activities) or their impacts (Hoagland et al., Reference Hoagland, Anderson, Kaoru and White2002). For instance, limiting shellfish harvesting closures or varying the timing of shellfish or finfish harvesting could be useful strategies to reduce the impacts of HABs, but these actions also may have associated costs in terms of product marketability. Wessells et al. (Reference Wessells, Miller and Brooks1995) found that the occurrence of paralytic shellfish poisoning in blue mussels from the Canadian maritimes, leading to illnesses and subsequent harvest closures, adversely affected the market for mussels from Maine. Whitehead et al. (Reference Whitehead, Haab and Parsons2003) found that, after consumer demand for seafood contracted upon learning of a HAB-induced fish kill, mandatory seafood inspection programmes were more effective in restoring consumer confidence and expanding demand than broad public assurances of seafood safety.
Experience suggests that closure strategies in shellfish production areas could be beneficial over the long term, despite leading to losses in the short term. For example, this approach has been applied recently in the northern areas of Hokkaido and Tohoku (Japan) with some benefits. The region provides more than the 60% of the scallop aquaculture in Japan, accounting for about 500 thousand tonnes per year with a value of ~ JPY84.0 billion (~US$700.0 million USD). Paralytic and diarrhetic shellfish poisoning events had caused the complete closure of shellfish harvesting in the 1980s, resulting in dramatic economic impacts (Imai et al., Reference Imai, Shimada, Shinada, Baba, Kanamori, Sato, Kuwahara, Miyoshi, Tada, Hirano, Miyazono, Itakura, Trainer and Yoshida2014). Another example is from the Galician region of NW Spain. The Rías are a highly productive ecosystem that sustain a production of 0.2–0.3 million tonnes of mussels (Mytilus edulis) per year. Bans on harvesting can last up to 9 months (particularly in Dinophysis bloom hotspot areas), when DSP biotoxin levels exceed European Union regulatory thresholds (e.g. Blanco et al., Reference Blanco, Correa, Muñíz, Mariño, Martín and Arévalo2013). NW Europe has the highest incidence of diarrhetic shellfish poisoning (DSP) in the world, and intensive monitoring of biotoxins attempts to ensure that any closures of shellfish harvesting are limited to the minimum time needed.
Some data about the economic losses caused by ichthyotoxic HAB species in aquaculture are shown in Box 2. In most cases, fish-killing HAB species (e.g. Karenia mikimotoi, K. brevis, Prymnesium spp., Chattonella spp.) produce biotoxins with neurotoxic, haemolytic or cytotoxic effects on fishes. Some HAB species have been linked directly to massive fish kills, often at aquaculture sites. In 2003, losses in European coastal waters were estimated at more than €800 million in fisheries associated with HABs (Scatasta et al., Reference Scatasta, Stolte, Granéli and van Ierland2003; note that these are global data, including finfish and shellfish wild harvests and aquaculture). Analogous losses in the USA were conservatively estimated at around US$4.0 million annually, adjusted for inflation (Hoagland et al., Reference Hoagland, Anderson, Kaoru and White2002). Kim (Reference Kim, Granéli and Turner2006) reported HAB-related aquaculture losses in Japan of more than US$1.0 billion annually. Recent estimates were presented at the PICES (North Pacific Science Organization) ‘Workshop on Economic Impacts of Harmful Algal Blooms and Aquaculture’ (Trainer & Yoshida, Reference Trainer and Yoshida2014). From 2006 to 2012, the total economic losses in farmed fish and shellfish production was estimated as ~US$94.0 million for Korea, Japan and China. Note that many of these estimates are lost sales (gross revenues); they should be regarded as over-estimates of true economic losses because they do not account for the avoided costs of not fishing. Where commercial wild harvest fisheries are managed inadequately and therefore economically or biologically over-exploited (a common occurrence), lost net economic values, representing actual declines in human welfare, are likely to be a small fraction of reported lost sales.
There may be special impacts of high biomass HABs on the operation of desalination plants, which would result in the disruption of water production and significant economic losses. For instance, in 2008 and 2009, at least five seawater desalination plants were closed in the United Arab Emirates (UAE) due to a dense bloom of the ichthyotoxic Cochlodinium polykrikoides in the Arabian Gulf and Gulf of Oman that lasted for more than 8 months (Richlen et al., Reference Richlen, Morton, Jamali, Rajan and Anderson2010; Anderson & McCarthy, Reference Anderson and McCarthy2012). In this particular case, algal biomass clogged the filtration systems and reverse osmosis membranes.
Impacts of HABs on non-market, passive use values of marine ecosystems
Certain HABs have noxious, even lethal, consequences on marine organisms and ecosystems (Box 2), thereby potentially leading to passive value losses or the degradation of ecosystem services.
Some taxa produce particular toxic compounds that, given their high concentrations in the water when the species bloom, have been involved in mass mortalities of wild fauna. According to the US National Oceanic and Atmospheric Administration (NOAA), more than 50% of all Unusual Mortality Events (UMEs) of wild animals (turtles, dolphins, manatees, whales, birds; e.g. Scholin et al., Reference Scholin, Gulland, Doucette, Benson, Busman, Chavez, Cordaro, DeLong, De Vogelaere, Harvey, Haulena, Lefebvre, Lipscomb and Van Dolah2000), could have been due to microalgal biotoxins, although it is evident that this is not a new phenomenon and is also a part of the natural ecosystem functioning. There are reports of mortalities of wild fish associated with HABs that date back to the latter part of the 19th century (Whitelegge, Reference Whitelegge1891). In 1968, mortalities of sand eels (Ammodytes spp.) and an estimated 80% of the breeding population of shag (Phalacrocorax aristotelis) coincided with a bloom of Alexandrium tamarense off the north-east coast of the UK (Adams et al., Reference Adams, Seaton, Buchanan and Longbottom1968; Coulson et al., Reference Coulson, Potts, Deans and Fraser1968). White (Reference White1984) documented four cases of fish kills associated with saxitoxins. Other historical examples include the deaths of 19 humpback whales off Cape Cod (USA), and probably the deaths of pygmy, dwarf sperm and North Atlantic right whales in the same region, as well as Southern right whale mortalities in Peninsula Valdés (Argentina). More recently, Scholin et al. (Reference Scholin, Gulland, Doucette, Benson, Busman, Chavez, Cordaro, DeLong, De Vogelaere, Harvey, Haulena, Lefebvre, Lipscomb and Van Dolah2000) reported the deaths of over 400 California sea lions (Zalophus californianus) along the central Californian coast during May and June 1998. Coincident with these mortalities, a bloom of Pseudo-nitzschia australis was reported and domoic acid was detected in planktivorous fish and in sea lion body fluids.
Jessup et al. (Reference Jessup, Miller, Ryan, Nevins, Kerkering, Mekebri, Crane, Johnson and Kudela2009) reported extensive marine bird mortality in the Pacific North-west (Monterey Bay, California) in winter 2007 coinciding with a bloom of the non-toxic dinoflagellate Akashiwo sanguinea. Affected birds had a slimy yellow-green material on their feathers, and they were diagnosed as severely hypothermic. This dinoflagellate excretes high amounts of organic matter that accumulate at the sea surface. In this case, the sea foam contained surfactant-like proteins, which destroyed the waterproofing and insulative characteristics of the bird feathers.
Ostreopsis blooms constitute a particular case of an emerging harmful event that is a growing problem in coastal environments. Since the end of the 20th century in temperate waters (e.g. in the Mediterranean, Brazilian and New Zealand coasts), blooms of this benthic dinoflagellate genus have been linked to damage to marine fauna (i.e. mussel mortalities, loss of spines and death of sea urchins, loss of one or more arms in sea stars, and coral bleaching) and subsequent alterations of the coastal ecosystems (Sansoni et al., Reference Sansoni, Borghini, Camici, Casotti, Righini and Rustighi2003; Simoni et al., Reference Simoni, Gaddi, Paolo and Lepri2003; Shears & Ross, Reference Shears and Ross2009). In some cases, the invertebrate community of the affected area has not recovered to date (Vila et al., Reference Vila, Arin, Battocchi, Bravo, Fraga, Penna, Reñé, Riobó, Rodríguez, Sala, Camp, de Torres and Franco2012). Coinciding with these effects, high cell concentrations of O. cf. ovata (mainly in the Mediterranean) or O. siamensis (mainly in New Zealand) were recorded in the water column. Also, highly visible distinctive rusty-brown coloured mucilaginous films covered the reef, macroalgae and other sedentary organisms, or floated in the water column or on the surface. Oxygen depletion seems to be the most likely cause of the observed effects. Direct toxicity to the fauna (via ingestion of Ostreopsis through the food web) cannot be ruled out, given that palytoxin analogues (i.e. ostreocin and ovatoxin) have been isolated from certain macrofauna at other Mediterranean sites (Aligizaki et al., Reference Aligizaki, Katikou, Nikolaidis and Panou2008, Reference Aligizaki, Katikou, Milandri and Diogène2011; Amzil et al., Reference Amzil, Sibat, Chomérat, Grossel, Marco-Miralles, Lemee, Nézan and Séchet2012; Biré et al., Reference Biré, Trotereau, Lemée, Delpont, Chabot, Aumond and Krys2013).
High-biomass Phaeocystis blooms also have been linked to the deterioration of water quality in some coastal regions of the North Atlantic and the Mediterranean (e.g. Lancelot & Mathot, Reference Lancelot and Mathot1987; Arin et al., Reference Arin, Guillén, Segura-Noguera and Estrada2013). This species produces macroscopic colonies embedded into a mucilaginous matrix. The decay of the blooms results in brownish foams containing 3-dimethylsulphoniopropionate (DMSP), which is responsible for bad odours (Liss et al., Reference Liss, Malin, Turner and Holligan1994). Although this may result indirectly in the loss of recreational activities, no economic assessment has been undertaken yet, and no direct harm to humans has been reported.
Finally, some studies have suggested that certain HAB species also could have subtle impacts on ecosystems, by altering fundamental physiological and biological processes (e.g. feeding behaviour, life stages, survival strategies, reproductive capacity) in other organisms of food webs, likely through allopathic effects (e.g. Fistarol et al., Reference Fistarol, Legrand and Granéli2003; Tillmann, Reference Tillmann2003; Granéli & Hansen, Reference Granéli, Hansen, Granéli and Turner2006). These particular effects are not easy to detect, however, and the underlying mechanisms of damage to other components of the food webs remain difficult to understand (Landsberg, Reference Landsberg2002; Shumway et al., Reference Shumway, Allen and Boersma2003). This is due, in part, to the spatial and temporal variability of HAB events and also to the difficulty of isolating their impacts from those associated with other environmental and/or anthropogenic factors (e.g. increased temperature, wave action, habitat destruction). Both long-term field studies and experimental approaches in the laboratory are required to ascertain the direct and indirect impacts of HABs in the different components of the ecosystems.
The challenges of estimating the economic costs of HABs
As explained, HABs can lead to economic losses associated with the costs of treating human illnesses, closures of commercial and recreational shellfisheries, mortalities of fish in commercial finfish aquaculture operations, declines in coastal recreation and tourism, and additional investments in environmental monitoring and the prevention, control or mitigation of blooms or their consequences (Hoagland et al., Reference Hoagland, Anderson, Kaoru and White2002). Hoagland & Scatasta (Reference Hoagland, Scatasta, Granéli and Turner2006) estimated economic effects (adjusted for inflation into 2015 US$) in the order of US$1.0 billion per year in Europe and US$100.0 million per year in the United States. The European estimate was influenced largely by estimated losses to coastal tourism, which is affected mostly by high biomass, noxious (blooms causing high foam volumes, discolouration of the ocean, noxious odours, or beach closings), but not necessarily toxic, HABs. Any estimates of the scale of the economic effects of HABs should be considered very rough approximations, even in areas where they have been relatively well studied, including the USA and Europe. Estimates in other parts of the world, particularly in developing countries, are much more speculative and uncertain. The development of comprehensive and consistent estimates of losses in economic welfare as a consequence of HABs remains a clear priority for future research.
The reasons why economic estimates often are uncertain include the wide variety of methodologies used to compile the assessments, some of which produce estimates of economic measures that may not be strictly comparable (e.g. sales, indirect or induced impacts, consumer or producer surpluses, agency budgets); the wide variety of physical and economic effects; the episodic and often unpredictable nature of blooms of different types in many areas; and the wide ranges of responses of humans to mitigate the adverse impacts, including, most importantly, switching to next-best alternative activities or foods. Moreover, these reasons tend to be context-dependent, because of differences in the capabilities of human communities for responding to HABs as a natural hazard. For example, shellfish management measures are well-practiced in many parts of the developed world, such as in Maine or Florida in the USA, but often non-existent or difficult to enforce in parts of the developing world, such as in the coastal bays of the Bohol and Visayan Seas in the Philippines. Morbidities and mortalities from shellfish poisonings, particularly among the underprivileged, are common in the latter but now exceedingly rare in the former (but see Reich et al. (Reference Reich, Lazensky, Faris, Fleming, Kirkpatrick, Watkins, Ullman, Kohler and Hoagland2015) for a modern counter-example of neurotoxic shellfish poisonings from Florida).
Other so-called non-market, ‘passive’ uses of the coastal and marine ecosystems, such as for the conservation of protected species (including marine mammals and sea turtles), can in theory be affected by HABs. Even if favoured species or unique ecosystems are not used directly, humans may experience a sense of loss that might be evaluated in economic terms. Studies of passive value losses due to the adverse effects of HABs on protected species or ecosystems are almost non-existent. One reason for the lack of estimates of effects on passive use values is that, although individuals may become ill or die as a consequence of HABs, such as the deaths of endangered West Indian manatees (Trichechus manatus) due to Karenia brevis blooms in south-western Florida, the populations often are largely unaffected by the mortalities (in essence, K. brevis blooms could be viewed as one of many natural sources of mortality). Similarly, the media often report on ‘large-scale’ finfish kills resulting from HABs, but the impacts on fish biomass and the growth rates of fish stocks in the wild often are minor or imperceptible.
Of greater concern to users of coastal and marine ecosystems is the loss of access to commercial or recreational fisheries, especially to high-valued shellfish stocks, such as oysters, scallops, clams, mussels, crabs, or marine snails, or to areas used to grow shellfish. Even in such cases, commercial fishermen (a market-based, direct use) tend to relocate or to switch fisheries or occupations, thereby mitigating substantial economic losses. Recreational fishermen (a non-market, direct use) choose another target or another pastime. Further, seafood consumers also can switch among food options. When switching occurs, other fisheries, recreational destinations, or food providers may benefit. Because the latter typically are not ‘first-best’ choices, economic losses are ipso facto incurred in all cases, but the human behavioural switching response clearly serves to blunt the impacts. Here, policies to improve the communication of risks, say through the widespread publication of the locations of closures or the species affected, which facilitates human switching, also may help significantly to mitigate economic losses. Finally, shellfish rarely are adversely affected by blooms, and one consequence of this ‘immunity’ to biotoxins is that areas closed to shellfish harvesting may become de facto fishery reserves, helping to conserve stocks, provided that the biotoxin is naturally metabolized or lost after the bloom disappears.
Social impacts of the health risks caused by toxic HABs. The case of ciguatera fish poisoning
Within the human communities whose revenue is based on aquaculture and shellfish activities, HABs may have adverse social impacts including damaged reputations, decreased incomes, employment losses, and longer-term changes in seafood consumption patterns (Magnien, Reference Magnien2001). There has been very little study of these other human dimensions, and many social impacts remain mostly conjectural and unexplored (Bauer et al., Reference Bauer, Hoagland, Leschine, Blount, Pomeroy, Lampl, Scherer, Ayres, Tester, Sengco, Sellner and Schumacker2009).
In the case of the ciguatera fish poisoning (CFP), it has been associated with the loss of a traditional food source, losses in commercial fishing businesses, losses of tourism, increased costs of medical care, and high costs of monitoring and management (Epstein & Rapport, Reference Epstein and Rapport1996). Fear of CFP can lead to reduced fishing in coral lagoons (Dewailly et al., Reference Dewailly, Chateau-Degat and Suhas2008), and to a corresponding increased reliance on pelagic fish or on a less healthy diet of lower quality, imported, canned fish or red meat. This new diet, combined with reduced levels of physical exercise, may be one factor contributing to the trend of increasing obesity among Pacific Islanders, accompanied by a rising prevalence of chronic diseases (e.g. diabetes, hypertension and cardiovascular diseases) in indigenous Pacific populations (Lewis & Ruff, Reference Lewis and Ruff1993).
Ciguatera fish poisoning outbreaks could harm the economies of tropical islands, many of which are highly dependent on local reef fisheries for subsistence, export products, and tourism. Reduced fishing effort due to endemic ciguatera has been blamed for losses of traditional fishing knowledge, as new generations abandon artisanal or commercial fishing. One example concerns Rarotonga, an island located in the southern Cook Islands that exhibits the world's highest incidence of ciguatera poisoning. On Rarotonga, the per-capita fresh fish consumption decreased from 149 g d−1 in 1989 to 75 g d−1 in 2006, due to concerns about CFP (Rongo & van Woesik, Reference Rongo and van Woesik2012). Consequently, over the same period, the consumption of alternative proteins, particularly imported meats, increased. The direct loss in value of marketable goods from commercial fisheries amounted to NZ$0.8 million (about US$0.5 million) per year, and the approximate costs associated with dietary shifts amounted to NZ$1.0 million (about US$0.7 million) per year. With a decline in cases of ciguatera poisoning in recent years, fresh fish has returned to the menus of Rarotongans, and per-capita fresh fish consumption increased to 104 g d−1 in 2011.
In a discussion of the effects of HABs on public health, it should be highlighted that the management of tropical waters for ciguatera fish poisoning is not well-developed. In these regions, fishermen usually are able to continue to access areas where finfish may have bioaccumulated ciguatoxin, leading to circumstances in which CFP illnesses may become widespread. Given that, researchers and managers may wish to prioritize characterizing the extent of the CFP problem in tropical regions and developing ways to alleviate it.
PRESENT AND FUTURE CHALLENGES, STRATEGIES AND OPPORTUNITIES
HABs are natural phenomena that have almost certainly occurred for thousands of years before recorded history (Mudie et al., Reference Mudie, Rochon and Levac2002). To humans, HABs constitute a problem worldwide, but the idea that HABs might be prevented from occurring is not a viable option. However, improvements in our understanding of HAB dynamics during the last 40 years have led to insights for designing strategies to mitigate their impacts. Successful strategies consider the present situation and the future prospects of the potential spread and increase in HAB occurrence as a result of climate and other environmental changes. Common strategies can be adapted to address specific, local problems in different parts of our world. Despite some limitations, new advances in technology and coordinated international research efforts may help to improve the management of the impacts of HABs.
Improving monitoring and research needs to forecast and predict HAB events
Monitoring programmes for HAB species and their toxins implemented in different areas of the world have clearly reduced fatal poisonings in humans (e.g. Davidson & Bresnan, Reference Davidson and Bresnan2009). For example, current monitoring programmes of the European Union are driven by its Food Hygiene Regulations (EC) No. 853/2004 and (EC) No. 854/2004, which require Member States to monitor both for biotoxin concentrations in shellfish tissues and the presence of marine biotoxin-producing phytoplankton in coastal waters. Due to different capacities for undertaking local risk assessments, there are local differences in the implementation of these monitoring activities. In general, these programmes have been successful in safeguarding human health, but continued refinement will be needed in the future as environmental conditions shift (particularly climate warming) and human uses of the coastal ocean change.
Currently missing are programmes of robust monitoring in developing countries where they are often at best rudimentary, despite increasing exports of seafood products to other regions (Reich et al., Reference Reich, Lazensky, Faris, Fleming, Kirkpatrick, Watkins, Ullman, Kohler and Hoagland2015). Despite their success, established monitoring programmes are not without technical, logistical or cost-related limitations (DeGrasse & Martinez-Diaz, Reference DeGrasse, Martinez-Diaz, Cabado and Vieites2012; Hess, Reference Hess, Cabado and Vieites2012; Suzuki & Watanabe, Reference Suzuki, Watanabe, Cabado and Vieites2012). Since their inception, improvements have been linked to advances in technologies related to sampling, taxonomic and biotoxin analyses, and observing systems.
Light microscopy constitutes the most widespread monitoring tool for harmful algal species, but it is time consuming to employ, and it requires specialized training and expertise. Development of quantitative biomolecular tools to facilitate monitoring could improve monitoring. Given the number of different organisms that have to be tracked, and the lack of fully quantitative molecular tools for their enumeration, monitoring agencies cannot currently replace light microscopy as the primary tool. Maintaining and supporting the training of microscopists in the identification and quantification of HABs species is necessary for successful monitoring.
While the ability to detect the presence of harmful microalgal species in coastal waters can provide an excellent early warning of the likely occurrence of biotoxins in, for example, shellfish (e.g. Trainer & Suddleson, Reference Trainer and Suddleson2005), many uncertainties remain. These include the fact that the presence of potentially toxic organisms is not always linked to toxicity in shellfish. For example, in Scottish waters, morphologically indistinguishable toxic and non-toxic Alexandrium tamarense cells have been known to occur simultaneously (Touzet et al., Reference Touzet, Davidson, Pete, Flanagan, McCoy, Amzil, Maher, Chapelle and Raine2010). Furthermore, known-toxic organisms are not always detected efficiently due to their benthic character, fragility when preserved, or small size. For example, the azaspiracid producer Azadinium spinosum measures only 7 × 12 µm. The link with its produced biotoxin was established recently in 2007 (Tillmann et al., Reference Tillmann, Elbrächter, Krock, John and Cembella2009), only after standards for biotoxin analysis were isolated (Satake et al., Reference Satake, Shoji, Oshima, Naoki, Fujita and Yasumoto2002; Jauffrais et al., Reference Jauffrais, Kilcoyne, Séchet, Herrenknecht, Truquet, Hervé, Bérard, Nulty, Taylor, Tillmann, Miles and Hess2012; Kilcoyne et al., Reference Kilcoyne, Keogh, Clancy, LeBlanc, Burton, Quilliam, Hess and Miles2012) and biomolecular tools for tracking the organism in water were developed (Kilcoyne et al., Reference Kilcoyne, Jauffrais, Twiner, Doucette, Aasen, Sosa, Krock, Séchet, Nulty, Salas, Clarke, Geraghty, Duffy, Foley, John, Quilliam, McCarron, Miles, Silke, Cembella, Tillmann and Hess2014). Further, toxicity itself is not always well described. For example, not all of the biotoxins of many Gambierdiscus species have been described, and they may be present in complex mixtures in different individual organisms and blooms. Finally, toxicity may be associated with new, unexpected vectors or species. For example, in 2002, the consumption of puffer fish from Florida caused neurological disease in 21 people (Centers for Disease Control and Prevention (CDCP), 2002). Initially, based on patient symptoms and reported puffer fish ingestion, the outbreak was attributed to tetrodotoxin. A mouse bioassay confirmed the presence of a Na+ channel blocking toxin, tentatively identified as tetrodotoxin (usually associated with puffer fish), in unconsumed portions of fish. Direct measurement analyses (LC/MS, immunoassay, and receptor binding assay) confirmed the unexpected presence of saxitoxin in the fish samples, however, but not tetrodotoxin. Analysis of urine samples from the victims of the outbreak confirmed these findings. This was the first confirmed report of saxitoxin poisoning associated with puffer fish ingestion in North America (Landsberg et al., Reference Landsberg, Hall, Johannessen, White, Conrad, Abbott, Felwelling, Richardson, Dickey, Jester, Etheridge, Deeds, Van Dolah, Leighfield, Zou, Beaudry, Benner, Rogers, Scott, Kawabata, Wolny and Steidinger2006).
Concerning biotoxin detection, bioassays (i.e. the mouse bioassay) had been used traditionally as a fast way to detect the presence of toxins in potentially contaminated seafood, thus raising warnings and preventing the harvest of contaminated shellfish. Bioassays are associated with a number of technical and ethical issues (Hess et al., Reference Hess, Grune, Anderson, Aune, Botana, Caricato, van Egmond, Halder, Hall, Lawrence, Moffat, Poletti, Richmond, Rossini, Seamer and Serratosa Vilageliu2006), however, and led to their recent replacement with non-animal alternatives (i.e. cell tissue cultures; e.g. Van Dolah et al., Reference Van Dolah, Fire, Leighfield, Mikulski and Doucette2012) in many countries, particularly in Europe (European Commission, 2011). Research efforts are now focused on the development of multi-toxin methods for the detection of multiple groups of compounds in biosensor systems (Campbell et al., Reference Campbell, McNamee, Huet, Delahaut, Vilarino, Botana, Poli and Elliott2014, and references cited therein). The large number of compounds that need to be detected and an inability to validate new tools for detection mean that many monitoring agencies will need to continue to rely on either insensitive (and ethically questionable) mouse bioassays or on comparatively complex and expensive LC-MS/MS detection methods, neither of which can be implemented in situ.
A potentially useful sampling technique based on the passive capture of biotoxins by resins was developed by MacKenzie et al. (Reference MacKenzie, Beuzenberg, Holland, McNabb and Selwood2004). This method has been used successfully to trace biotoxins when the producing organisms are difficult to monitor due to their small size (e.g. Azadinium spp.) or their benthic habit (e.g. Prorocentrum lima and Vulcanodinium rugosum; Fux et al., Reference Fux, Biré and Hess2009; Zendong et al., Reference Zendong, Herrenknecht, Abadie, Brissard, Tixier, Mondeguer, Séchet, Amzil and Hess2014). The technique has not been standardized, however, and it will need further development for hydrophilic biotoxins. At present, the passive capture of biotoxins cannot easily be automated or adapted for in situ detection. Furthermore, ongoing refinement of its analytical methods will be required to meet the challenges of emerging biotoxins (Turner et al., Reference Turner, Higgins, Veszelovski, Payne, Davidson, Hungerford and Higman2015).
Issues arise with the existing technologies with the frequency of representative sampling of in situ biotoxins and the toxin-producing microalgae, which rarely takes place more than weekly, although higher frequency sampling may be desirable in some coastal waters. In the particular case of the benthic harmful species (e.g. Gambierdiscus, Ostreopsis, Prorocentrum lima), sampling techniques require refinements and standardization (e.g. Tester et al., Reference Tester, Kibler, Holland, Usup, Vandersea, Leaw, Lim, Jacob Larsen, Mohammad-Noor, Faust and Litaker2014). As yet automated, reliable, and affordable systems to detect harmful species or their biotoxins for routine monitoring in situ are lacking. While recently some promising techniques have been developed to detect biotoxin-producing organisms (e.g. Hess et al., Reference Hess, Amzil, Belin, Compere, Lassus, Menesguen, Pagou and Hallegraeff2012 and references cited therein; ICES, 2015), they are still viewed mainly as research tools and have seldom been used in high frequency in situ monitoring. This is the case of the Environmental Sample Processor (ESP; Greenfield et al., Reference Greenfield, Marin, Doucette, Mikulski, Jones, Jensen, Roman, Alvarado, Feldman and Scholin2008; Scholin et al., Reference Scholin, Doucette, Jensen, Roman, Pargett, Marin, Preston, Jones, Feldman, Everlove, Harris, Alvarado, Massion, Birch, Greenfield, Vrijenhoek, Mikulski and Jones2009) and the Imaging Flow Cytobot (IFCB; Olson & Sosik, Reference Olson and Sosik2007), two automated in situ monitoring platforms deployed in Monterey Bay, the Gulf of Maine, Puget Sound and the Gulf of Mexico. The ESP collects and processes water samples in situ, using sensitive and specific molecular assays to detect target HAB cells and toxins, and transmitting the data to the laboratory in near real time. The IFCB is an imaging flow cytometer that captures the high-resolution images and fluorescence characteristics of all plankton cells (in the 5–150 µm size range) at a high frequency (3 samples per hour); the data can also be sent to a laboratory in near real time (e.g. Campbell et al., Reference Campbell, Olson, Sosik, Abraham, Henrichs, Hyatt and Buskey2010, Reference Campbell, Henrichs, Olson and Sosik2013). At present, however, they have very high costs and cannot be deployed to monitor individual shellfish production areas. Although they cannot replace more traditional forms of monitoring at present efforts are being made to facilitate their use for this purpose. Fortunately, these sophisticated systems are useful tools for advancing an understanding of the links between HABs and ocean dynamics.
The adoption of monitoring technologies are affected strongly by economic costs (Frolov et al., Reference Frolov, Kudela and Bellingham2013). Some systems are relatively simple, comprising microscopic analysis of water samples for particular HAB species and analysis of shellfish tissues for the presence of biotoxins. Other programmes involve in situ monitoring of phytoplankton and model-based forecasts of bloom occurrence, such those carried out in the Gulf of Maine (e.g. Anderson et al., Reference Anderson, Keafer, Geyer, Signell and Loder2005a, Reference Anderson, Stock, Keafer, Nelson, McGillicuddy, Keller, Thompson, Matrai and Martinb; McGillicuddy et al., Reference McGillicuddy, Anderson, Lynch and Townsend2005; http://www.whoi.edu/main/topic/harmful-algae-red-tides) or in California Bay (e.g. Ryan et al., Reference Ryan, Chavez and Bellingham2005, Reference Ryan, Greenfield, Marin, Preston, Roman, Jensen, Pargett, Birch, Mikulski, Doucette and Scholin2011). According to Bernard et al. (Reference Bernard, Kudela, Velo-Suárez, Djavidnia, Cheung, Ott and Seeyave2014), the costs of monitoring could be on the order of 1 billion USD annually, accounting for 10% of the overall costs of HABs worldwide, estimated to be at 10 billion USD annually for marine and fresh waters. A monitoring system including satellite observations could be made more efficient by improving international cooperation in the collection, interpretation and sharing of Earth observation information, as conceived by the Global Earth Observation System of Systems (GEOSS, Fritz et al., Reference Fritz, Scholes, Obersteiner, Bouma and Reyers2008).
Monitoring, combined with operational oceanography and modelling, offers the hope of providing forecasts and early warnings and ultimately predictions of HAB events (e.g. GEOHAB, Reference McGillicuddy, Glibert, Berdalet, Edwards, Francks and Ross2011; Davidson, Reference Davidson2014). Achieving a predictive capability would require an adequate understanding of the ecophysiology of HAB species and the physical and chemical processes that influence the occurrence of the blooms, however. One problem is that, in many contexts, such as remote sensing from satellites, HAB species do not exhibit characteristics that distinguish them from other phytoplankters. Our understanding of the many different processes (biological, ecological, physical, chemical, meteorological) that control HAB dynamics and the dynamics of microalgae in general is incomplete, making their parameterization in numerical models extremely difficult. To date, progress has been constrained by the complexities of biological interactions and the resulting difficulties in developing ecological models. One example is the HAB operational forecast (HAB-OFS) developed by NOAA for Florida and Texas (Stumpf et al., Reference Stumpf, Litaker, Lanerolle and Tester2008; http://tidesandcurrents.noaa.gov/hab/). HAB-OFS combines satellite imagery, field observations, models, public health reports and buoy data to help forecast K. brevis blooms. Another NOAA effort for forecasting Pseudo-nitzschia has incorporated input from stakeholder communities in the state of Washington (Brown et al., Reference Brown, Green, Hickey, Jacobs, Lanerolle, Moore, Schwab, Trainer, Trtanj, Turner, Wood, Wynne, Morain and Budge2012). In the Gulf of Maine, a coupled physical/biological model (e.g. He et al., Reference He, McGillicuddy, Keafer and Anderson2008) predicts the transport of Alexandrium fundyense cells, controlled by plume advection and wind forcing. Statistically based models (Anderson et al., Reference Anderson, Siegel, Kudela and Brzezinski2009; Lane et al., Reference Lane, Raimondi and Kudela2009) have successfully hindcast Pseudo-nitzschia blooms in Monterey Bay and Chesapeake Bay. Finally, conceptual models, such as for Phaeocystis blooms in Vietnam (Hai et al., Reference Hai, Lam and Dippner2010) and Dinophysis acuminata (Velo-Suárez et al., Reference Velo-Suárez, González-Gil, Pazos and Reguera2014) in the Rías of north-western Spain, have provided new insights into understanding the dynamics of blooms and designing preventive strategies.
Sustained fundamental research could help generate a better understanding of HAB dynamics, to inform and improve monitoring programmes, and to design methods to mitigate the impacts of HABs on human health and well-being.
Better quantification and prevention of the impacts on human health: HAB-related disease surveillance
Determining the true incidence of HAB-related diseases remains a very significant challenge. The lack of experience of professionals in the public health and medical communities with patients exposed to marine biotoxins (e.g. Pennotti et al., Reference Pennotti, Scallan, Backer, Thomas and Angulo2013) has led to incorrect diagnoses and failures to report illnesses. Inexperience, in turn, likely results in the under-reporting of HAB-related disorders, even in areas where such diseases are endemic. Furthermore, in general, there is a lack of coordination between marine biotoxin monitoring and public health surveillance activities.
Under-reporting due to erroneous diagnosis is common and widespread, and is related, in part, to the lack of diagnostic tools. For instance, the symptoms of diarrhetic shellfish poisoning (DSP) are non-specific, and, except during exceptional outbreaks, neither seafood consumers nor their clinicians may recognize the incidence of DSP illness (e.g. Davidson et al., Reference Davidson, Tett, Gowen, Hester and Harrison2011; Taylor et al., Reference Taylor, Mcintyre, Ritson, Stone, Bronson, Bitzikos, Rourke, Galanis and Team2013). Taylor & Harrison (Reference Taylor, Harrison, Taylor and Trainer2002) query that ‘[n]o diarrhoeic shellfish poisoning has been diagnosed in humans in British Columbia, but, given its resemblance to diarrhoea caused by bacterial contamination (Vibrio haemolyticus, in particular), would DSP be detected without testing specifically for okadaic acid or dinophysistoxin?’ Indeed, routine methods for the detection of either biotoxins or biomarkers in human tissues or fluids are virtually absent. [In 2011, nine years after Taylor had queried whether diarrhetic shellfish poisoning would be detected in the absence of testing for okadaic acid or dinophysistoxin, 62 illnesses of diarrhetic shellfish poisoning were reported in British Columbia. Changes were made to the shellfish monitoring programme following this outbreak to include more stringent testing for DSP toxins (Taylor et al., Reference Taylor, Mcintyre, Ritson, Stone, Bronson, Bitzikos, Rourke, Galanis and Team2013).]
Diagnostic tools for saxitoxins in human urine have recently been developed (Johnson et al., Reference Johnson, Zhou, Statler, Thomas, Cox, Hall and Barr2009). A biomarker for low-level domoic acid exposure of zebra-fish and sea lions (Lefebvre et al., Reference Lefebvre, Frame, Gulland, Hansen, Kendrick, Beyer, Bammler, Farin, Hiolski, Smith and Marcinek2012), is a promising technology for identifying such biomarkers for humans. The verification of ciguatoxins in contaminated seafood has been particularly challenging. Recent advances suggest that an effective and reliable method will soon be available. Under the auspices of the International Atomic Energy Agency (IAEA), a radio-labelled receptor binding assay (RBA) was developed for ciguatoxin, and this assay has been tested successfully in the Caribbean and the Pacific at IAEA-UNESCO-IOC sponsored training workshops (Tester, personal communication). A more recent advance is a fluorescent RBA method that compares favourably with the radio-labelled RBA approach. The fluorescent method is analogous to a technique implemented for brevetoxins (e.g. McCall et al., Reference McCall, Jacocks, Baden and Bourdelais2012), in that no radioactivity is involved, making it less expensive to use, and avoiding hazardous waste disposal costs that can be significant, for example, in the Pacific islands.
HAB-associated illnesses are a public health issue, and, accordingly, public health surveillance could be coordinated with environmental monitoring. Until now, epidemiological studies typically have been conducted after clusters of disease outbreaks have occurred (although unfortunately usually without long-term follow-up to explore the chronic health effects from acute exposures). New multidiscipliary approaches could help to identify relevant bloom events, measure biotoxins in seawater (and in some cases air) and seafood, and identify symptoms in humans and other animals associated with exposures. In addition, communications with potentially affected communities could be conducted to promote the understanding that a particular symptom may be the result of exposure to a HAB biotoxin.
An example of such an approach can be found in the Gulf of Mexico, where the link between Karenia brevis blooms and human exposures and health effects, particularly to contaminated aerosols, was established. These efforts facilitated the successful understanding and mitigation of the human health risks associated with these events. This large and costly initiative included coordination among physicians, toxicologists, ecologists, stakeholders (including businesses, public health agencies, Poison Information Centres) and end-users (Fleming et al., Reference Fleming, Kirkpatrick, Backer, Bean, Wanner, Dalpra, Tamer, Zaias, Cheng, Pierces, Naar, Abraham, Clark, Zhou, Henry, Johnson, Van de Bogart, Bossart, Harrington and Baden2005, Reference Fleming, Kirkpatrick, Backer, Walsh, Nierenberg, Clark, Reich, Hollenbeck, Benson, Cheng, Naar, Pierce, Bourdelais, Abraham, Kirkpatrick, Zaias, Wanner, Mendes, Shalat, Hoagland, Stephan, Bean, Watkins, Clarke, Byrne and Baden2011).
Another initiative with the same aim was the Harmful Algal Bloom-related Illness Surveillance System (HABISS) (http://www.cdc.gov/hab/surveillance.htm) that the US Centers for Disease Control and Prevention (CDC) and other public health and environmental organizations established to create a coordinated human, animal and environmental health surveillance network. The simultaneous collection of environmental and health data over time was considered to have helped public health practitioners identify long-term trends in HAB-related diseases in humans and animals. The CDC also has created a module within the National Outbreak Reporting System (NORS) to capture HAB-related illnesses. Beta-testing of the system began in the summer of 2015.
In Europe, similar initiatives, although at a smaller scale, have been established in the Mediterranean region to develop tools and strategies to manage the impacts of Ostreopsis blooms on human health before they can have a wider impact. Examples conducted at local scale include France (Tichadou et al., Reference Tichadou, Glaizal, Armengaud, Grossel, Lemée and Kantin2010; Lemée et al., Reference Lemée, Mangiajo, Cohu, Amzil, Blanfune, Chomerat, Ganzin, Gasparin, Grossel, Guidi-Guivar, Hoareau, Le Duf, Marr, Simo, Néza, Pedrott, Séche, Soliveres and Thibaut2012) and the Spanish Catalan coast (Vila et al., Reference Vila, Arin, Battocchi, Bravo, Fraga, Penna, Reñé, Riobó, Rodríguez, Sala, Camp, de Torres and Franco2012), and the Accord RAMOGE (http://www.ramoge.org) at Mediterranean region level (mainly on the coasts of Italy, Monaco, France and Spain).
HABs dynamics in the context of global climate change
Climate plays a fundamental role in the physical dynamics of the water masses and energy pathways in the ocean, which in turn modulate the biogeochemical fluxes and thus nutrient supplies to microorganisms at the bases of food webs (Beaugrand et al., Reference Beaugrand, Edwards and Legendre2010). Climate variability modulates ecological events (phenology) and ecosystem characteristics at different spatio-temporal scales (Longhurst, Reference Longhurst2007). As a result, the dynamics of HABs, as a natural phenomenon, are also influenced by climate variability.
The Earth's climate has changed continually over scales of millions of years, as evidenced by glacial and interglacial periods. Its climate also exhibits much shorter-term cyclical changes at multiannual to decadal and multi-decadal scales, such as those reflected in the El Niño Southern Oscillation (ENSO) and the North Atlantic Oscillation (NAO).
Photosynthetic organisms have evolved and adapted with changes occurring at these different temporal scales (e.g. Beardall & Raven, Reference Beardall and Raven2004). In the last 200 years, however, since the industrial revolution, human activities have impacted the Earth dramatically, mainly as a consequence of the increasing release of carbon dioxide (CO2), methane and nitrous oxides into the atmosphere. In particular, atmospheric CO2 concentration has increased from 280 ppm to >380 ppm at present, with values of 750–1000 ppm predicted by 2100 (IPCC, 2008), in comparison to fluctuations between 180 and 300 ppm during the previous 800,000 years.
This rapid and uncontrolled rate of increase in so-called greenhouse gases has been responsible for an augmentation in about 1°C of global temperature in the past 20–30 years (data from the Hadley Centre for Climate Prediction and Research; shown in figure 1 of Hallegraeff, Reference Hallegraeff2010), with a further rise of 2–4°C predicted over the next 100 years. Climate warming is already directly and indirectly impacting terrestrial and marine ecosystems. In the oceans, increased temperature in the upper layers can lead to changes in density which in turn affect the seasonal patterns of mixing, stratification and circulation, acidification, weakening or reinforcement of upwelling winds, and modification of the freshwater inflows to coastal regions (e.g. Bindoff et al., Reference Bindoff, Willebrand, Artale, Cazenave, Gregory, Gulev, Hanawa, Le Quéré, Levitus, Nojiri, Shum, Talley, Unnikrishnan, Solomon, Qin, Manning, Chen, Marquis, Averyt, Tignor and Miller2007). These changes may influence many ecological processes, including the occurrences of HABs. Given the impacts that HABs have on humans, there is now an urgent need to investigate the potential effects of climate warming on the occurrence of HABs. In addition, it will be important to develop tools for tracking and evaluating recent and future trends in HAB dynamics at both local and global scales.
The possible impacts of climate change on toxic marine HABs include: (1) range-changes in both warm- and cold-water species; (2) changes in abundance and toxicity; and (3) changes in the timing of the seasonal window of growth (e.g. Hays et al., Reference Hays, Richardson and Robinson2005; Moore et al., Reference Moore, Trainer, Mantua, Parker, Laws, Backer and Fleming2008; Hallegraeff, Reference Hallegraeff2010; Anderson et al., Reference Anderson, Cembella and Hallegraeff2012; Fu et al., Reference Fu, Tatters and Hutchins2012). On the other hand, the effects of climate change on other components of the food webs (e.g. predators, competitors, parasites) could modulate the impact of climate change on the occurrence, magnitude and duration of HABs. Some HAB species may benefit such that their impact on human health and wellbeing becomes more severe, whereas others may diminish in areas that are currently impacted (Hallegraeff, Reference Hallegraeff2010). Our ability to forecast the directions of change for toxic marine HABs is constrained by inadequate understanding of the interactions among multiple climate change variables and non-climate stressors in conjunction with inadequately designed experiments for investigating decadal- or century-scale trends (Hallegraeff, Reference Hallegraeff2010; Fu et al., Reference Fu, Tatters and Hutchins2012).
Few studies have investigated the effects of projected future climate change conditions on individual species or genera of toxin-producing marine microalgae. Of the few studies to date that have directly investigated future climate change effects on certain HAB species or genera, most have focused on the effects of warming. For example, the 2.5–3.5°C projected increase in sea surface temperature in the Caribbean over the coming century has been estimated to increase the incidence of CFP by 200–400% (Gingold et al., Reference Gingold, Strickland and Hess2014). In Puget Sound, warming has been projected to increase the seasonal window of growth for Alexandrium by ~30 days by 2040, allowing blooms to begin earlier in the year and to persist for longer periods (Moore et al., Reference Moore, Johnstone, Banas and Salathé2015). It is important to remember that these projections are primarily based on projected changes to water temperature. Other aspects of bloom ecology and oceanography, such as the location of nutrient availability, competition with other phytoplankton species, grazing, and infection by parasites, were not considered. Therefore, while these initial assessments of greenhouse gas-driven changes to HAB risk provide insight into potentially important climate pathways that are relevant for bloom development, more work is needed to understand better the interactive effects of drivers other than temperature on HAB occurrence.
An emerging body of work is also focusing on the effects of ocean acidification on marine HABs. Some experiments performed on Pseudo-nitzschia fraudulenta found significantly high production of the domoic acid neurotoxin in response to a combination of low pH and some other factor that limits growth (Sun et al., Reference Sun, Hutchins, Feng, Seubert, Caron and Fu2011; Fu et al., Reference Fu, Tatters and Hutchins2012; Tatters et al., Reference Tatters, Fu and Hutchins2012a, Reference Tatters, Schnetzer, Fu, Lie, Caron and Hutchinsb, Reference Tatters, Flewelling, Fu, Granholm and Hutchins2013), provided that the limiting resource was not needed for toxin synthesis. Saxitoxin (paralytic shellfish toxin) production increased with elevated temperature and increased CO2 supply (Kremp et al., Reference Kremp, Godhe, Egardt, Dupond, Suikkanen, Casabianca and Penna2012), although the response was highly strain specific. Much more work is needed to understand the effect of increasing ocean acidification on HAB species, and thus the potential effect on human health and the ecosystems under this scenario.
In order to evaluate future trends of HAB events, there is a need to establish the present baseline. Several studies report that the occurrence and geographic distribution of toxic marine HABs has increased in certain areas during the past few decades (Hallegraeff, Reference Hallegraeff1993, Reference Hallegraeff2010; Van Dolah, Reference Van Dolah2000; Lewitus et al., Reference Lewitus, Horner, Caron, Garcia-Mendoza, Hickey, Hunte, Huppert, Kudela, Langlois, Largier, Lessard, RaLonde, Rensel, Strutton, Trainer and Tweddle2012). Increased awareness, expanded and better monitoring and detection of toxic HABs, and the multiple effects and interactions of human activities (including, but not limited to, increased nutrient loading to coastal waters, aquaculture development and transport of seed stock, ballast water transport) and climate warming may have contributed to this putative increase (Smayda, Reference Smayda, Granéli, Sundstrøm, Edler and Anderson1990; Hallegraeff, Reference Hallegraeff1993, Reference Hallegraeff2010; Sellner et al., Reference Sellner, Doucette and Kirkpatrick2003; Gowen et al., Reference Gowen, Tett, Bresnan, Davidson, McKinney, Harrison, Milligan, Mills, Silke and Crooks2012). Direct observations of microalgae in coastal marine waters on timescales sufficient to evaluate climate change effects and to discriminate the role of other natural or anthropogenic forcings (e.g. nutrient loads) are typically lacking. This makes it difficult to establish whether the observed trends are global or local (Davidson et al., Reference Davidson, Gowen, Tett, Bresnan, Harrison, McKinney, Milligan, Mills, Silke and Crooks2012). While a few isolated time series do exist (e.g. Borkman & Smayda, Reference Borkman and Smayda2009; Kim et al., Reference Kim, Miller, McGowan and Carter2009), they have not yet been fully utilized to describe changes in the abundances of HAB species. In some cases, the available HAB data are from offshore, such as that generated by the Continuous Plankton Recorder (CPR) in the North Atlantic (Edwards et al., Reference Edwards, Reid and Planque2001, Reference Edwards, Johns, Leterme, Svendsen and Richardson2006; Hinder et al., Reference Hinder, Hays, Edwards, Roberts, Walne and Gravenor2012). Even though humans typically encounter HABs and their toxins at the coastal margins, these offshore time series provide valuable insights into the ecological responses of HAB species on timescales relevant to climate change. A small number of toxic HAB species leave a long-term record of their abundance in bottom sediments as microfossils (Dale et al., Reference Dale, Edwards, Reid, Granéli and Turner2006) which could be used to explore time-series of bloom events. On the basis of similarities in the sediment cyst records in the Pacific and Atlantic regions of Canada, Mudie et al. (Reference Mudie, Rochon and Levac2002) concluded that climate change (including surface temperature and storminess) was a main factor stimulating blooms.
Reported illnesses are also an indicator of toxic HABs (Van Dolah, Reference Van Dolah2000); however, seafood is often consumed at long distances from where harvested. Unless robust information on the source of the seafood consumed is reported, it is difficult to identify potential relationships between food-borne HAB-related diseases and climate. Because most of the HAB-related diseases are associated with the consumption of contaminated shellfish, time series of HAB toxins in shellfish tissues provide valuable data for evaluating long-term trends and relationships to climate and in some cases date back to the 1950s (Trainer et al., Reference Trainer, Eberhart, Wekell, Adams, Hanson, Cox and Dowell2003). The interactions of weather and climate are important for understanding patterns of disease, with climate determining the range of species and weather determining the timing and intensity of outbreaks (Dobson & Carper, Reference Dobson and Carper1993; Epstein, Reference Epstein2001).
At present, it is clear that we lack sufficient scientific understanding of climate-driven changes to nearshore marine environments, as well as HAB responses to these potential changes, to provide accurate predictions of future HAB occurrences in space and time. One option to improve understanding of these changes would be to facilitate multidisciplinary research and coordination including stakeholders and policy makers with the objective to mitigate the impacts of HABs on human health and well-being. The concern about this need motivates discussions and meetings in different fora, such as the Symposium on ‘HABs and Climate Change’ held in Göteborg, Sweden, 19–22 May 2015 (https://pices.int/meetings/international_symposia/2015/2015-HAB/scope.aspx).
Mitigation strategies for aquaculture
In Japan, several strategies have been adopted to minimize economic losses to aquaculture (Imai et al., Reference Imai, Shimada, Shinada, Baba, Kanamori, Sato, Kuwahara, Miyoshi, Tada, Hirano, Miyazono, Itakura, Trainer and Yoshida2014). In south-west Hokkaido's Funka Bay, for example, the scallop culturing industry has adapted to frequent occurrences of dinoflagellate blooms that produce the dinophysis toxin and paralytic shellfish poisoning (PSP), which typically occur in May and continue into the summer. Modified approaches to harvesting were necessitated, in part, by high PSP contamination in the 1980s that stopped shipments of all bivalves. Timing the scallop harvest every year from December to April avoids the impacts associated with the toxic bloom season. Scallops are harvested both for immediate fresh consumption, and they can be inventoried by boiling and freezing in order to provide a source of supply during the closed season.
In the Okhotsk Sea, recent occurrences of paralytic shellfish poisoning contamination of scallops have been predicted on the basis of movements of the Soya Warm Current. The strength of this current prevents the transport of Alexandrium tamarense, which typically resides in the offshore water masses of the Okhotsk Sea, to locations such as Monbetsu and Abashiri on the northern coast of Hokkaido. Adaptive harvesting in response to these forecasts is an effective way to avoid the impacts of toxic blooms on the bivalve aquaculture industry. This strategy is effective because of the fundamental understanding of local HAB dynamics.
Some strategies to mitigate the impacts of HABs on aquaculture may have been discounted prematurely and are worth revisiting. The use of clay to flocculate and remove toxic cells at some aquaculture sites in Asia (Sengco & Anderson, Reference Sengco and Anderson2004) is one example. This approach could be studied further to explore tradeoffs between the economic losses associated with fish kills from HABs, the environmental consequences of clay dispersal, the costs of implementing the mitigation technology, and toxin accumulation in the benthos.
Communication with the public and improving literacy about oceans and HABs
Throughout history, humans have learned how to cope with natural phenomena, including HABs. In some areas, such as in the Pacific Islands (Chinain et al., Reference Chinain, Darius, Ung, Fouc, Revel, Cruchet, Pauillac and Laurent2010b), local traditional knowledge about ciguatera is functionally correct, albeit scientifically incomplete.
The Spanish explorer Alvar Núñez Cabeza de Vaca (1490–1558) recorded a place along the northern coast of Mexico in the Gulf of Mexico, probably not far from Apalache Bay, where indigenous peoples were unable to relate the passage of time to the movements of the sun and the moon, but instead marked the seasons by fishkills (Ferrando, Reference Ferrando1984), which could have been caused then, as now, by blooms of Karenia brevis.
To people making a living around the Galician Rías, red-tides (‘purgas de mar’) are familiar events, traditionally compared with menstruation, through which local waters are cleansed, usually in the autumn. It was general knowledge that it was unsafe to eat shellfish gathered when the ocean exhibited a reddish-brownish hue. When mass cultivation of mussels in the Galician Rías was undertaken by developers, who were ignorant of the blooms, the dispersal of toxic mussels became more frequent. One particularly extreme case occurred during the autumn of 1976 (Estrada et al., Reference Estrada, Sánchez and Fraga1984).
Fukuyo et al. (Reference Fukuyo, Imai, Kodama, Tamai, Taylor and Trainer2002) note that in Northern Japan, local folklore advises not to eat shellfish during runoff of snowmelt into the sea occurring in the early spring. In the UK and the USA, there is a similar folklore (O'Connor Reference O'Connor2008; Whyte Reference Whyte2013): one should eat shellfish only during months that are spelled with the letter ‘r’, i.e. avoid shellfish consumption the summer months (May to August) when HABs are more likely to occur (although bacterial contamination of the food cannot be discounted). Perhaps, as Fukuyo et al. (Reference Fukuyo, Imai, Kodama, Tamai, Taylor and Trainer2002) suggest, such folklore has arisen because:
this indicates that toxin contamination of shellfish has repeatedly occurred almost every year over a long time, leading to many tragedies among the local people. Community education resulted in self-regulating behaviour to avoid high-risk fish species and certain fishing locations.
Successful communication about HABs and their effects can be problematic for many different reasons. In part, this is due to the complexity of HAB phenomena, which are currently difficult to predict and to prevent or control. Most public health and medical personnel do not have in-depth knowledge about HABs and their potential effects on human health and wellbeing. Nowadays, many coastal communities are not fully informed of the public health risks of HABs, however, which can result in a phenomenon known as the ‘halo effect’, where human activities unrelated to the HAB hazards are scaled back or discontinued (Wessells et al., Reference Wessells, Miller and Brooks1995; Whitehead et al., Reference Whitehead, Haab and Parsons2003; Parsons et al., Reference Parsons, Morgan, Whitehead and Haab2006). A common form of the halo effect is a reduction in the consumption of all types of seafood, regardless of the actual risks of consuming phycotoxins. Other forms of the halo effect can influence commercial and recreational fishing behaviour and coastal tourism. Consequently, it would be beneficial to improve the scientific messages about HABs to the many and diverse end users and stakeholders.
Fortunately, there are now examples of interesting and potentially successful dissemination and early warning activities related to safeguarding human health from HAB-related toxic episodes. For example, the US National Oceanographic and Atmospheric Administration (NOAA) produces HAB Bulletins which provide forecasting of HABs for managers in the Gulf of Mexico (for Karenia brevis), in the Pacific North-west (for toxic Pseudo-nitzchia) and the Great Lakes (for cyanobacteria). The bulletins incorporate oceanographic modelling, satellite imagery and on-the-ground monitoring, as well as other information (e.g. real-time reports from lifeguards and managers). These materials were developed with stakeholder engagement and input, so that the outputs are actually useful for and used by the people (such as beach managers) who need timely information. Another example of appropriate communication was the implementation of a toll-free, 24/7-telephone number at the Miami Poison Information Centre staffed by poison specialists trained to discuss HAB exposure and illnesses in Florida. This resource was signposted on information panels and brochures placed in beaches, hotels and other tourist venues. Another useful aspect of this centralized information resource was that the poison control phone calls also could be incorporated into HAB human health surveillance activities to increase case reporting (Fleming et al., Reference Fleming, Kirkpatrick, Backer, Walsh, Nierenberg, Clark, Reich, Hollenbeck, Benson, Cheng, Naar, Pierce, Bourdelais, Abraham, Kirkpatrick, Zaias, Wanner, Mendes, Shalat, Hoagland, Stephan, Bean, Watkins, Clarke, Byrne and Baden2011).
Examples in Europe include HAB bulletins that are produced weekly in both Ireland and Scotland for the aquaculture industries in these countries (http://www.marine.ie/Home/site-area/data-services/interactive-maps/weekly-hab-bulletin; http://www.somuchtosea.co.uk/news/ bulletin_for_shellfish_farmers.aspx). In the Baltic, the Swedish Meteorological and Hydrological Institute Service offers reports and almost real-time information about the algae situation in the Baltic obtained from satellite data (http://www.smhi.se/en/weather/sweden-weather/1.11631). In the NW Mediterranean, the RAMOGE Accord is working to communicate with the public and stakeholders, communicating through brochures and web pages, with dissemination activities about the occurrence of the Ostreopsis blooms and their impacts on human health and the environment, and searches to provide tools to manage them (http://www.ramoge.org/fr/ostreopsis_ovata.aspx). RAMOGE also fosters the coordination of international research around the understanding and management of these events in the affected countries.
International coordination: the GEOHAB and GlobalHAB programmes
HABs are a global challenge which needs to be addressed at local levels by implementing a broad global vision. During the last few decades, much work has been accomplished to understand HAB dynamics with the ultimate aim of predicting their occurrences and mitigating their impacts. Studies and monitoring of toxic phytoplankton and biotoxins have been conducted at local, national and regional levels, each with their own particular resources. In addition, international and interdisciplinary cooperation has been invaluable in advancing the science of HABs. As an international exemplar, this cooperative research has been fostered from 2000 to 2014, by the GEOHAB programme, ‘Global Ecology and Oceanography of Harmful Algal Blooms’ (http://www.geohab.info), with the financial support of SCOR (Scientific Committee on Oceanic Research) and IOC/UNESCO (Intergovernmental Oceanographic Commission of UNESCO). As stated in its Science Plan (GEOHAB, Reference Glibert and Pitcher2001): ‘[t]he scientific goal of GEOHAB was to improve the ability to predict HABs by determining the ecological and oceanographic mechanisms underlying their population dynamics, and integrating biological, chemical, and physical studies supported by enhanced observation and modelling systems.’
GEOHAB acted as an umbrella and catalyst for organizing scientific research on HABs. Moreover, GEOHAB provided a common and interconnecting aim for individual needs and efforts. The initiatives sponsored by GEOHAB, including open science meetings, specific workshops, and training activities, were publicized as reports, books and special issues in international journals (http://www.geohab.info).
At the termination of GEOHAB in Reference Berdalet, Bernard, Burford, Enevoldsen, Kudela, Magnien, Roy, Tester, Urban and Usup2014, the international scientific community agreed that a coordinated research approach to HABs was beneficial and still necessary (see GEOHAB, Reference Berdalet, Bernard, Burford, Enevoldsen, Kudela, Magnien, Roy, Tester, Urban and Usup2014). Starting in 2015, a new programme, GlobalHAB, will build on the GEOHAB Science Plan, incorporating the present challenges and opportunities that international research on HABs requires (Figure 3). Taking multidisciplinary and international perspectives, GlobalHAB will integrate key aspects of climate warming and global change on HABs within the context of the field of ‘oceans and human health.’
CONCLUSIONS
Microalgal blooms are a natural part of the seasonal cycle of the marine ecosystems around the world. They are key components of the structure and dynamics of the oceans and thus sustain the benefits (food supply, recreation, commerce, livelihood,) that humans obtain from this aquatic environment.
However, some microalgal blooms can be harmful to human health, impact valuable fisheries, and degrade other marine and coastal ecosystem services. The impacts of these HABs comprise acute and chronic health effects in humans, financial losses from contaminated seafood, mortalities of farmed fish, reduced coastal tourism and altered socio-cultural aspects. The losses due to HABs of passive values that humans place on protected species and extraordinary marine ecosystems are more difficult to quantify. There is evidence in some littoral areas that human pressures may have increased the occurrence of HABs. Further, global warming and changes in the climate regime also could affect HAB occurrences and toxicity, although forecasting the possible trends is still speculative.
At the beginning of the 21st century, with expanding human populations, particularly in coastal and developing countries, there is an urgent need to prevent and mitigate the impacts that HABs pose on human health and wellbeing.
Because HABs are natural phenomena, it is not possible to prevent their occurrence. However, the scientific research conducted over the last four decades, with the support of stakeholders, policymakers and the general public, has improved the understanding of HAB dynamics.
The achieved insights allow defining key priorities and designing strategies to mitigate the HABs impacts. In this paper, we have summarized part of the present knowledge and available tools to address this general objective. Around the world, at varying levels of government, certain promising efforts are being attempted to mitigate HAB impacts. These efforts include:
• Integrating both ecosystem and human health monitoring for HAB impacts. This involves maintaining or expanding existing HAB and biotoxin monitoring, and implementing new monitoring programmes where necessary. It also includes the monitoring of physico-chemical and meteorological variables to help ascertain the real effects of climate and other environmental changes on HAB occurrences and their impacts.
• Maintaining and reinforcing initiatives and local and international policies to reduce human pressures on the marine environment that may increase the occurrence of HABs and the severity of associated events.
• Developing an increased public “ocean literacy” and expanded engagement with coastal and ocean stakeholders.
We have also identified key research needs that, if addressed, could greatly enhance our understanding over HABs and their impacts on health. These questions include:
• Ascertaining real trends in ciguatera fish poisoning incidence, and provide informed projections of potential future trends.
• Ascertaining the risks of new emerging HABs and biotoxins (e.g., azaspiracid food-borne poisonings, BMAA and neuromuscular diseases, and respiratory irritation in new areas impacted by Ostreopsis blooms).
• Investigating the responses of HABs (especially regarding toxin production) to multifactorial physico-chemical climate drivers and the potential of marine microalgae to adapt genetically and phenotypically to the unprecedented rapidity of current climate and other environmental changes. This will require multidisciplinary collaboration and appropriately scaled experimental designs.
• Investigating possible interconnections between socio-cultural aspects of human wellbeing and HAB events.
• Advancing in the estimation of the economic costs of HAB events.
Overall, we emphasize that collaborative research across natural and social scientific disciplines, as for example, the Florida Red Tide Research Group and GEOHAB, can lead to significant advances in our understanding of HABs, helping to develop approaches at local and global levels to lessen their impacts on public health and human wellbeing.
FINANCIAL SUPPORT
The research was funded in part by the UK Medical Research Council (MRC) and UK Natural Environment Research Council (NERC) for the MEDMI Project; the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Environmental Change and Health at the London School of Hygiene and Tropical Medicine in partnership with Public Health England (PHE), and in collaboration with the University of Exeter, University College London and the Met Office; and the European Regional Development Fund Programme and European Social Fund Convergence Programme for Cornwall and the Isles of Scilly (University of Exeter Medical School).
EB was supported by the CTM2014-53818-R project, from the Spanish Government (MINECO). KDA was in receipt of funding from the BBSRC-NERC research programme for multidisciplinary studies in sustainable aquaculture: health, disease and the environment. P. Hess was supported by Ifremer (RISALTOX) and the Regional Council of the Pays de la Loire (COSELMAR). Porter Hoagland was supported by the US National Science Foundation under NSF/CNH grant no. 1009106.
This work contributes to the implementation of the objectives of the SCOR and IOC/UNESCO funded programmes GEOHAB and GlobalHAB.