Overall, there will be about 2·1 million incident breast cancer (BC) cases diagnosed in 2018. This disease is the most frequently diagnosed cancer in many countries (154 of 185) and is also the leading cause of cancer death in over 100 countries( Reference Bray, Ferlay and Soerjomataram1 ). According to 2015 Spanish statistics, BC is the most commonly diagnosed cancer among women( Reference Galceran, Ameijide and Carulla2 ). Despite the recent data suggesting a decrease in the death rate due to BC, the high prevalence of this disease highlights an unmet need for prevention efforts, which include the promotion of lifestyle factors. Thus, preventive strategies that may help tackle this condition are of great importance from a public health perspective.
One lifestyle factor that has been suggested to be related to BC is diet. Particularly, previous literature has described a potential association between adherence to the Mediterranean dietary pattern and a reduction in the risk of developing BC( Reference Toledo, Salas-Salvadó and Donat-Vargas3 ). Among others, the Mediterranean diet is characterised by a high consumption of fresh fruits and vegetables, nuts and extra-virgin olive oil( Reference Trichopoulou, Martínez-González and Tong4 ). All these foods share in common a high content of polyphenols( Reference Gorzynik-Debicka, Przychodzen and Cappello5 ).
Polyphenols are a wide and diverse family of more than 8000 natural substances present in plant foods (cereals, vegetables, fruits, nuts, legumes, etc.) and beverages (wine, tea, coffee, cocoa, etc.) characterised by a structure of at least one aromatic ring with one or more hydroxyl groups attached with a large variability( Reference Del Rio, Rodriguez-Mateos and Spencer6 ). They are considered as responsible for some of the properties of these products, such as their sensory and nutritional qualities. Even though they are not essential for short-term well-being as it is the case for vitamins, they have shown favourable effects on the incidence of chronic diseases including cancer( Reference Del Rio, Rodriguez-Mateos and Spencer6 ). Over the last years, in vitro and in vivo studies have demonstrated bioactivity for many individual polyphenols which includes specific and non-specific properties such as their antioxidant effects, cell cycle arrest, apoptosis stimulation, autophagy promotion, anti-inflammatory effects, anti-oestrogenic mechanisms, aromatase inhibiting properties, human epidermal growth factor receptor 2 (HER2) down-regulation and plasma membrane receptors modulation, reduced angiogenesis, reversal of glycolytic metabolism, epigenetic modifications, BC stem cell control, epithelial mesenchymal transition interruption and interference with cancer progression( Reference Gorzynik-Debicka, Przychodzen and Cappello5 , Reference Losada-Echeberría, Herranz-López and Micol7 , Reference Mocanu, Nagy and Szöllősi8 ).
Several prospective cohort studies assessed the association between single polyphenol subtypes and the risk of BC( Reference Buck, Zaineddin and Vrieling9 , Reference Grosso, Godos and Lamuela-Raventos10 ). Nevertheless, evidence on total polyphenol intake and the risk of BC in prospective studies is scarce( Reference Touvier, Druesne-Pecollo and Kesse-Guyot11 ). In addition, dietary sources of polyphenol intake may vary across different populations. This is why we aimed to assess whether total polyphenol intake and also intake of main polyphenol subtypes were associated with BC risk in a Mediterranean cohort of university graduates (‘Seguimiento Universidad de Navarra’ (SUN) project).
Methods
Study population
The ‘SUN’ project (Universidad of Navarra follow-up project) is an ongoing, multipurpose and prospective cohort with open enrolment, which started in December 1999 and includes more than 22 000 Spanish university graduates. It aims to assess different associations between diet and lifestyles and the incidence of several chronic diseases and mortality. The study design, methods and cohort profile have been published in detail elsewhere( Reference Carlos, De La Fuente-Arrillaga and Bes-Rastrollo12 ). Until 1 December 2016, a total of 22 564 participants had completed a baseline questionnaire, from which 13 843 were female participants. As we needed at least one of the biennial follow-up questionnaires, we included in the analysis women recruited before 1 March 2014 (n 13 645).
For the analysis, we excluded women without follow-up (n 1286, retention rate 91 %), prevalent BC cases (n 102), women with a daily energy intake out of the pre-defined limits (below 2092 or beyond 14644 kJ/d) (n 1345), and those who attained menopause before 35 years of age (n 199). Finally, our sample size was 10 713 women (Fig. 1).
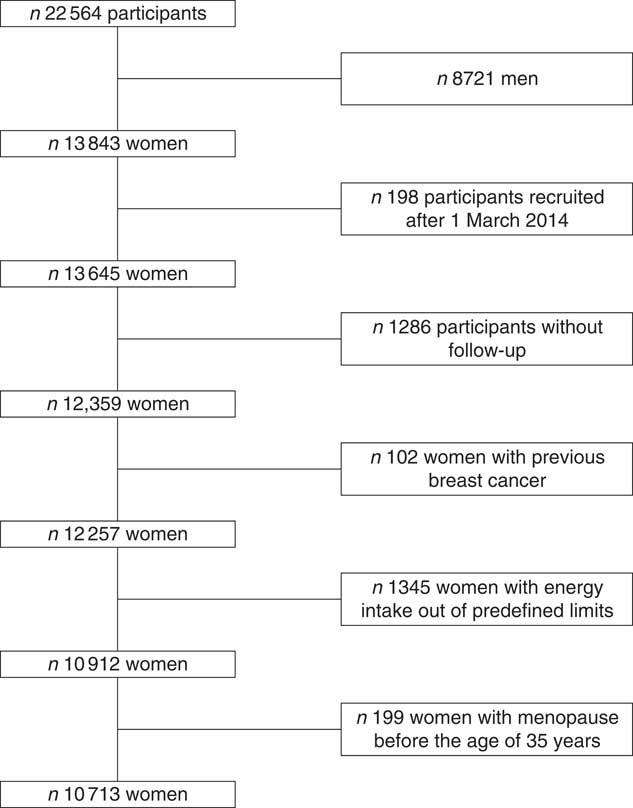
Fig. 1 Flowchart of participants recruited in the Seguimiento Universidad de Navarra project, 1999–2016.
Regarding the informed consent of potential participants, we duly informed these potential candidates of their right to refuse to participate in the SUN study or to withdraw their consent to participate at any time without reprisal, according to the principles of the Declaration of Helsinki. Special attention was given to the specific information needs of individual potential candidates as well as to the methods used to deliver their information and the feedback that may receive in the future from the research team. After ensuring that the candidate had understood the information, we sought their potential freely given informed consent, and their voluntary completion of the baseline questionnaire. These methods were considered by our Institutional Review Board as to imply an appropriately obtained informed consent.
Polyphenol intake and dietary assessment
Information on diet was collected from a previously validated 136-item FFQ at baseline( Reference Martin-Moreno, Boyle and Gorgojo13 ). Consumption frequencies were grouped into nine categories (never or seldom; 1–3 times/month; once weekly; 2–4 times/week; 5–6 times/week; once daily; 2–3 times/d; 4–6 times/d; 6 or more times per d) according to the typical portion size included for each item. We multiplied typical portion sizes by consumption frequency for each food item to calculate the daily food consumption. For different foods which were assessed together as one single item of the FFQ, we applied a weighted average using weights according to the typical relative frequency of consumption in the Spanish population( 14 ).
Data on the polyphenol content in foods were collected from the Phenol-Explorer database (www.phenol-explorer.eu)( Reference Neveu, Perez-Jimenez and Vos15 ) and expressed in mg/100 g for solid foods and oils or mg/100 ml for beverages. Certain polyphenols are present in foods as aglycone’s equivalents which refer to the core flavonoid, phenolic acid, lignan or stilbene, without the combined sugars or polyols.
The data used to estimate polyphenol intake represent the results of normal-phase HPLC for most phenolic compounds. Chromatography following basic or acid hydrolysis was applied for cereals and seeds (lignans in all foods, ellagic acid in walnuts and hydroxycinnamic acids in cereals, legumes and olives). Reverse phase HPLC was used for proanthocyanidins when values were not available by normal phase chromatography( Reference Pérez-Jiménez, Neveu and Vos16 ).
The accordance between food items in the FFQ and the Phenol-Explorer database was evaluated with the following steps: (1) foods with solely traces or without polyphenols were removed, (2) if foods were not included in Phenol-Explorer (leek, thistle and honey), we used the US Department of Agriculture database( Reference Bhagwat, Haytowitz and Wasswa-Kintu17 ), (3) items on the FFQ comprising more food components such as recipes and processed foods were divided according to their ingredients, (4) retention factors from Phenol-Explorer were used to consider the impact of food processing and cooking on polyphenol content values( Reference Bognár18 , Reference Rothwell, Medina-Remón and Pérez-Jiménez19 ) and (5) several products containing refined wheat flour were introduced in the list of foods (i.e. pizza, pastries) and polyphenol content was obtained from their wheat flour contents.
Finally, we calculated daily food consumption for the items included in the FFQ and matched this information with Phenol-Explorer database on polyphenol content of each reported food (April 2018). Total polyphenol intake was calculated as the sum of all individual classes of polyphenol intake (flavonoids, lignans, stilbenes, phenolic acids, alkylphenols, tyrosols, hydroxybenzaldehydes, hydroxybenxoketones, hydroxycoumarins and methoxyphenols) from all food sources found as stated in this process. We also calculated dietary intake of specific major subclasses of polyphenols, concretely, flavonoids, lignans, stilbenes and phenolic acids( Reference Pérez-Jiménez, Neveu and Vos16 ).
Ascertainment of incident breast cancer cases
Incident cases of BC were defined as the primary end point for this work. Participants completed follow-up questionnaires biennially in which they were asked to report incident BC cases. If a participant self-reported an incident case of BC, this participant was asked for a medical record that included this diagnosis, and the diagnosis was confirmed by a trained oncologist. When the medical record to confirm the diagnosis was not yet available, BC cases were considered for the analyses as probable incident cases. Subjects’ next of kin reported fatal cases to our research team. For participants lost to follow-up or with unknown causes of death, we consulted the National Death Index to identify deceased cohort members and to obtain the cause of death. When the cause of death in the official death certificate was BC, we considered this as a confirmed case.
We stratified our analyses by pre- or postmenopausal BC. Information on age at menopause was collected in the baseline questionnaire and updated after 16 years of follow-up. In the study population, for women lacking information on age at menopause, we established postmenopausal status according to the 75th percentile of age at menopause (52 years of age)( Reference Shivappa, Sandin and Lof20 ). When assessing premenopausal BC as outcome, we excluded those women who reported having had menopause before study inception and considered time at risk from baseline to age of menopause or date of turning 52 years, whichever occurred first. When assessing postmenopausal BC as outcome, we considered women to be at risk if they were postmenopausal at baseline. We also took into consideration for postmenopausal BC risk ascertainment the observation time of initially premenopausal women of whom we knew they had attained menopausal at a certain time during follow-up – started counting time at risk from that point on – or once they turned 52 years during follow-up.
Evaluation of covariates
We used the Spanish food composition tables to derive the nutrient composition of diet( Reference Mataix21 , Reference Moreiras, Carbajal and Cabrera22 ). From the baseline questionnaire, we also obtained the information about participants’ anthropometric measures, lifestyles, medical history and socio-demographic. Physical activity was assessed with a previously validated questionnaire( Reference Martínez-González, López-Fontana and Varo23 ). We estimated metabolic equivalents (MET) for each participant to obtain MET-h/week scores. We had previously validated the accuracy of self-reported weight and height for the calculation of BMI in a subsample of this cohort( Reference Bes-Rastrollo, Pérez Valdivieso and Sánchez-Villegas24 ).
Statistical analysis
Total and individual classes of polyphenol intake were adjusted for total energy intake using the residual method( Reference Willett25 ) and participants were grouped into tertiles.
We described baseline characteristics of our participants with means and standard deviations for continuous variables and percentages for categorical variables across tertiles of total polyphenol intake.
Person-years of follow-up were calculated for each participant from the date of completion of the baseline questionnaire to the date of BC diagnosis, the date of death, or the date of return of the last follow-up questionnaire, whichever occurred first. To assess the risk of BC by tertiles of total and individual classes of polyphenol intake (flavonoids, lignans, stilbenes and phenolic acids)( Reference Pérez-Jiménez, Neveu and Vos16 ), we ran Cox proportional hazard regression models with updated diet and covariates. Results are expressed as hazard ratios (HR) and 95 % CI, considering the lowest tertile as the reference category and with age as underlying time variable. In an attempt to control for potential confounding factors, which were selected based on previous literature, we used successive degrees of adjustment: (1) adjustment for age as underlying time variable and calendar year of recruitment in the cohort as stratification variable, (2) additional adjustment for height (continuous), years at university (continuous), number of relatives with history of BC (three categories), smoking status (never smoker, former smoker and current smoker), physical activity (MET-h/week, continuous), alcohol intake (g/d, continuous), BMI (three categories), age of menarche (five categories), age of menopause (three categories), number of pregnancies of more than 6 months (continuous), pregnancy before the age of 30 years (dichotomous), lifetime breast-feeding (continuous), use of hormone replacement therapy (dichotomous) and its duration (continuous) and (3) additional adjustment for diabetes (dichotomous), total energy intake (kJ/d, continuous) and adherence to the traditional Mediterranean diet (continuous)( Reference Trichopoulou, Costacou and Bamia26 ). In postmenopausal women, we additionally adjusted for the time between recruitment and menopause.
We conducted tests of linear trend, assigning to each category of the total polyphenol intake its tertile-specific median and using the resulting variable as continuous in the above-mentioned models.
We checked the proportional hazard assumption with the Grambsch–Therneau test of the scaled Schoenfeld residuals( Reference Royston and Lambert27 ) and introducing polyphenol intake as a time-varying covariate in the model.
We also calculated the main sources of polyphenol intake as well as the main sources of variability in polyphenol intake in our cohort for both individual foods and food groups (online Supplementary Table S1).
To calculate the contribution of each food item (or group of them) to the between-person variability in polyphenol intake, we conducted nested regression analyses after a stepwise selection algorithm. The contribution of each food group is shown in the cumulative R 2 change. Furthermore, we estimated their contribution related to total polyphenol intake as the mg consumed from each food group divided by the total polyphenol intake (%).
Considering that the overall risk of BC in the SUN project was 1·5 % and that we had 3571 women in each tertile, our sample size would allow us to detect absolute risk differences of 0·75 % in BC between extreme tertiles with a statistical power of 80 % and assuming a two-sided α error of 5 %.
All P values were two tailed and a P value <0·05 was deemed as statistically significant. All analyses were performed with Stata/SE 15·0.
Results
Median polyphenol intake was 662 (interquartile range: 482–893) mg/d. Table 1 shows the main characteristics of the 10 713 women included in our cohort according to the tertiles of polyphenol intake. We observed that women included in the third tertile were older, more physically active, had a higher alcohol intake, the percentage of pregnancies before 30 years old was higher and consisted of a higher proportion of current smokers and a higher percentage of premenopausal women. Characteristically, we observed a higher time of exposure to hormone replacement therapy for women in the lowest tertile of polyphenol intake. The mean age of the participants at recruitment was 34·7 (sd 10·6) years.
Table 1 Baseline characteristics from female participants in the Seguimiento Universidad de Navarra project according to tertiles of polyphenol intake, 1999–2014 (Mean values, standard deviations and percentages)
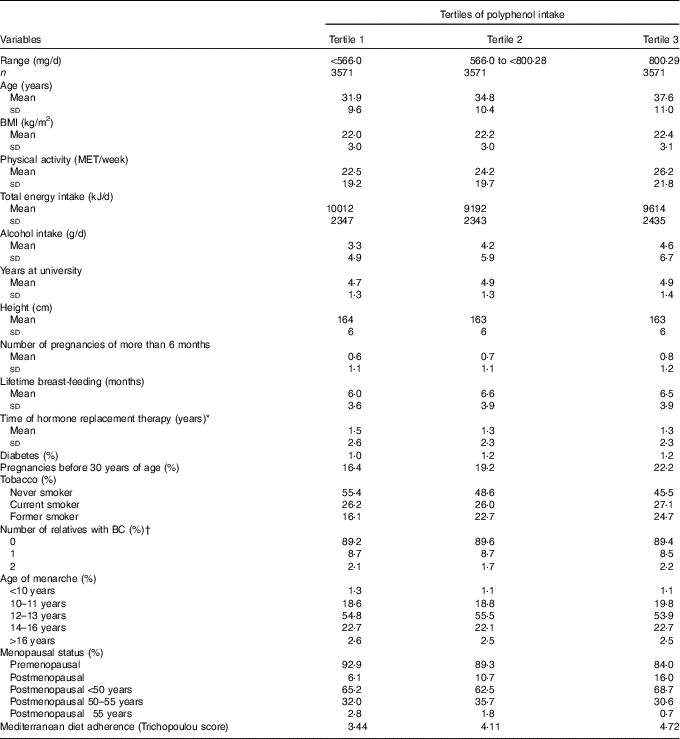
MET, metabolic equivalents; BC, breast cancer.
* Only for postmenopausal women.
† Information from mother, sister and both grandmothers was collected.
Total polyphenol intake and risk of breast cancer
During a median follow-up time of 10·3 years and 105 847 person-years at risk, 168 probable incident cases of BC were registered, out of which 100 were confirmed.
Regarding total polyphenol intake and probable BC cases, we found no significant association between total polyphenol intake and overall BC risk, neither for confirmed nor for probable cases (Table 2 and online Supplementary Table S2). When we stratified according to menopausal status, we observed that postmenopausal women within the highest tertile of total polyphenol intake showed a significantly lower risk of BC when probable cases were considered (HRT3 v. T1 0·35; 95 % CI 0·17, 0·73; P for trend=0·005) (online Supplementary Table S3) or when we considered only confirmed cases of BC (Table 3) with an HR of 0·31 (95 % CI 0·13, 0·77; P for trend=0·010) for the highest v. the lowest third of total polyphenol intake.
Table 2 Confirmed breast cancer cases across tertiles of total polyphenol intake in the Seguimiento Universidad de Navarra project (Hazard ratios (HR) and 95 % confidence intervals)
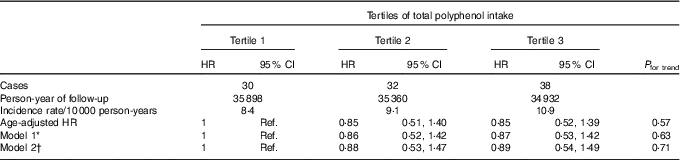
Ref., reference.
* Model 1: additional adjustment for height, number of relatives with history of breast cancer (three categories), smoking status (never smoker, former smoker and current smoker), physical activity (metabolic equivalent-h/week, continuous), alcohol intake (g/d, continuous), BMI (three categories), age of menarche (five categories), age of menopause (three categories), number of pregnancies of more than 6 months (continuous), pregnancy before the age of 30 years (dichotomous), months of breast-feeding (continuous), use of hormone replacement therapy (dichotomous) and its duration (continuous) and years at university (continuous).
† Model 2: additional adjustment for diabetes (dichotomous), total energy intake (kJ/d, continuous) and Mediterranean diet adherence (continuous).
Table 3 Confirmed breast cancer cases for each tertile of polyphenols in the Seguimiento Universidad de Navarra project among pre- and postmenopausal women (Hazard ratios (HR) and 95 % confidence intervals)
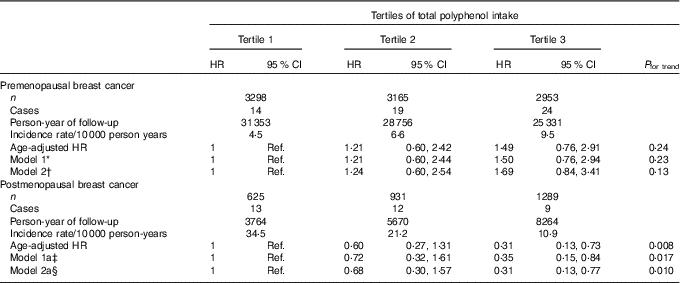
Ref., reference.
* Model 1: additionally adjusted for height (continuous), number of relatives with history of breast cancer (three categories), smoking status (never smoker, former smoker and current smoker), physical activity (metabolic equivalent-h/week, continuous), alcohol intake (g/d, continuous), BMI (three categories), age of menarche (five categories), number of pregnancies of more than 6 months (continuous), pregnancy before the age of 30 years (dichotomous), months of breast-feeding (continuous), use of hormone replacement therapy (dichotomous) and its duration (continuous) and years at university (continuous).
† Model 2: model 1 additionally adjusted for diabetes (dichotomous), total energy intake (kJ/d, continuous) and Mediterranean diet adherence (continuous).
‡ Model 1a: model 1 additionally adjusted for time since recruitment (continuous) and age of menopause (three categories).
§ Model 2a: model 2 additionally adjusted for time since recruitment (continuous) and age of menopause (three categories).
No significant associations were observed for premenopausal women (Table 3 and online Supplementary Table S3).
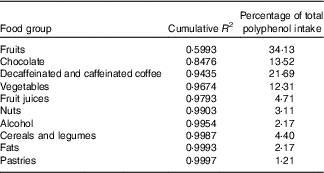
The contributions of different foods to the variability of total polyphenols intake are shown in Table 4. Individual foods that most contributed to total polyphenol intake were coffee (17·08 %), chocolate (13·52 %) and cherries (11·78 %) and to total polyphenol variability cherries, chocolate and apples. On the other hand, when we considered food groups – defined in online Supplementary Table S1 – those that contributed most to total polyphenol intake were fruits (34·13 %), decaffeinated and caffeinated coffee (21·69 %) and chocolate (13·52 %) and those that contributed most to total polyphenol variability were fruits, chocolate and decaffeinated and caffeinated coffee (Table 5).
Table 4 Sources of variability (cumulative R 2) and main sources (%) in total polyphenol intake according to each food included in the FFQ
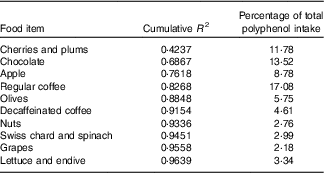
Table 5 Sources of variability (cumulative R 2) and main sources (%) in total polyphenol intake according to food groups
Individual classes of polyphenols and risk of breast cancer
When we assessed specifically the association between flavonoids, stilbenes, lignans and phenolic acids (Fig. 2), we observed a significant inverse association between phenolic acid intake and postmenopausal BC. None of the other classes of polyphenols was associated with total, premenopausal or postmenopausal BC.
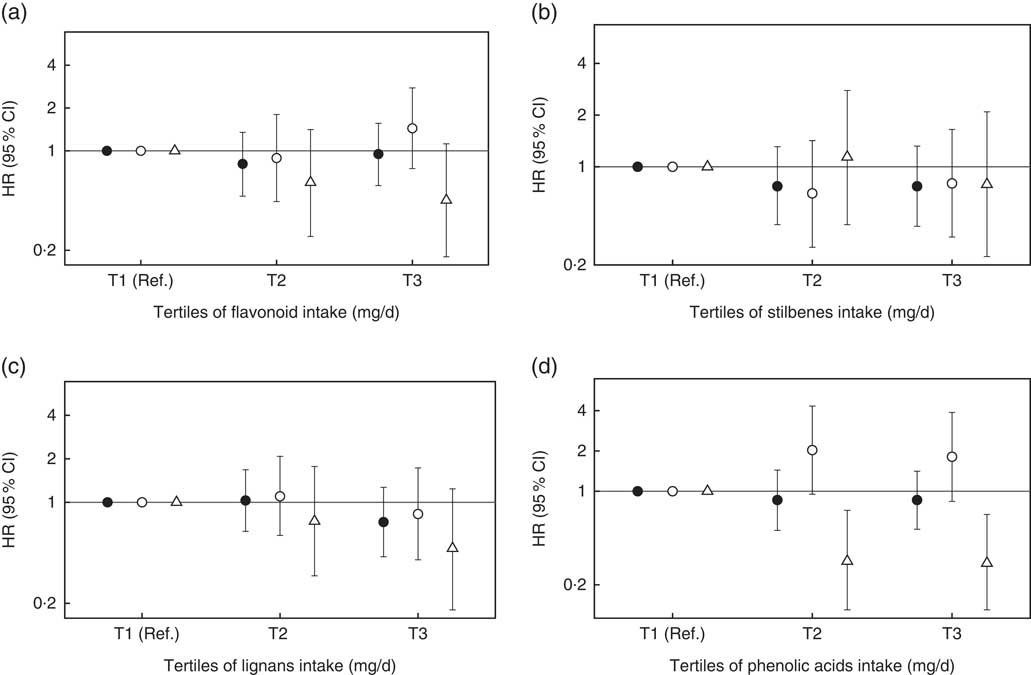
Fig. 2 Hazard ratios (HR) and 95 % confidence intervals for the association between classes of polyphenols intake and risk of breast cancer in the Seguimiento Universidad de Navarra project, 1999–2016. Ref., reference. All tertiles (T) are adjusted for height (continuous), number of relatives with history of breast cancer (three categories), smoking status (never smoker, former smoker and current smoker), physical activity (metabolic equivalent-h/week, continuous), alcohol intake (g/d, continuous), BMI (three categories), age of menarche (five categories), number of pregnancies of more than 6 months (continuous), pregnancy before the age of 30 years (dichotomous), months of breast-feeding (continuous), use of hormone replacement therapy (dichotomous) and its duration (continuous), years at university (continuous), diabetes (dichotomous), total energy intake (kJ/d, continuous) and Mediterranean diet adherence (continuous). For postmenopausal women, we further adjusted for time since recruitment (continuous) and age of menopause (three categories). ![]() , Overall women ((a) P
for trend=0·969, (b) P
for trend=0·454, (c) P
for trend=0·231 and (d) P
for trend=0·589);
, Overall women ((a) P
for trend=0·969, (b) P
for trend=0·454, (c) P
for trend=0·231 and (d) P
for trend=0·589); ![]() , premenopausal women ((a) P
for trend=0·197, (b) P
for trend=0·751, (c) P
for trend=0·606 and (d) P
for trend=0·221);
, premenopausal women ((a) P
for trend=0·197, (b) P
for trend=0·751, (c) P
for trend=0·606 and (d) P
for trend=0·221); ![]() , postmenopausal women ((a) P
for trend=0·239, (b) P
for trend=0·515, (c) P
for trend=0·126 and (d) P
for trend=0·006).
, postmenopausal women ((a) P
for trend=0·239, (b) P
for trend=0·515, (c) P
for trend=0·126 and (d) P
for trend=0·006).
Discussion
In this prospective cohort study, we found no overall association between total polyphenol intake and BC risk. By menopausal status, we observed an inverse association between total polyphenol intake and postmenopausal BC risk, even after adjustment for potential confounders. As far as specific classes of polyphenols are concerned, we observed an inverse association between phenolic acids intake and postmenopausal BC risk. No further associations were observed for other subclasses of polyphenols.
To our knowledge, there is no representative study of the Spanish population in which average polyphenol intake has been estimated. In the Prevención con Dieta Mediterránea (PREDIMED) trial, conducted in Spain, in which people at high cardiovascular risk were recruited, mean polyphenol intake was 820 (sd 323) mg/d( Reference Tresserra-Rimbau, Medina-Remón and Pérez-Jiménez28 ). In the European Prospective Investigation into Cancer and Nutrition study, Zamora-Ros et al. ( Reference Zamora-Ros, Knaze and Rothwell29 ) reported a median total polyphenol intake for the Mediterranean countries in Europe of 1011 mg/d, which was the lowest in their study, in comparison with non-Mediterranean and UK health-conscious groups. The median intake for population >30 years and University graduates was 1150 and 1275 mg/d, respectively. Thus, polyphenol intake in our cohort was lower than that in previous studies.
Evidence on the association between total polyphenol intake and BC is scarce. Consistent to our results, in the French SUpplémentation en VItamines et Minéraux Anti-oXydants (SU.VI.MAX) cohort( Reference Touvier, Druesne-Pecollo and Kesse-Guyot11 ), no association was observed between total polyphenol intake and BC. Nevertheless, the authors did not report results stratified by postmenopausal status. On the other hand, in our context, the traditional Mediterranean dietary pattern is rich in fruits and vegetables( Reference Trichopoulou, Martínez-González and Tong4 ) and, thus, in polyphenols. In line with our results, the inverse association between a higher adherence to the traditional Mediterranean dietary pattern and BC risk seems stronger for postmenopausal BC than for premenopausal BC( Reference Buckland, Travier and Cottet30 ).
As far as specific classes of polyphenols are concerned, epidemiological data on phenolic acids is sparse, and to the extent of our awareness, there is only one case–control study that has reported an inverse association between phenolic acids and a hormone-related cancer, concretely, prostate cancer( Reference Russo, Campisi and Di Mauro31 ). Chlorogenic acids, the most abundant phenolic acids, contained in foods such as coffee and tea, have demonstrated a hefty association with decreased risk of cancer mortality( Reference Grosso, Micek and Godos32 ) but also improved blood pressure alterations, glucose metabolism and decreased inflammation. Nevertheless, tea is not generally consumed in Southern Europe, albeit major sources of phenolic acids have been stated to be nuts and other foods allied to the Mediterranean diet( Reference Godos, Rapisarda and Marventano33 ). In our cohort, the main sources of between-person variability in phenolic acids were coffee, fruit, vegetables and nuts (data not shown).
Furthermore, in 2017, Grosso et al. ( Reference Grosso, Godos and Lamuela-Raventos10 ) published a large meta-analysis on flavonoid and lignan intake and cancer risk. They found no significant association between total flavonoid intake and BC risk when they pooled information from prospective cohort studies. Nevertheless, when only prospective cohort studies were pooled in that meta-analysis, a marginally significant inverse association was observed for total lignan intake (the main class of phytoestrogens in Western diets). Also, Rothwell et al. ( Reference Rothwell, Knaze and Zamora-Ros34 ) published a review in 2017 where they also described a reduction in BC risk with soya food consumption higher than 12 g/d or 1·5 times/week due to the oestrogenic activity of isoflavones, especially if the intake was in early life. Nevertheless, intake of soya products in Western populations, such as our population, is much lower (<1 mg/d). When menopausal status was considered, Buck et al. ( Reference Buck, Zaineddin and Vrieling35 ) found an inverse association between lignan intake and postmenopausal BC in their meta-analysis (seven studies, combined OR: 0·85, 95 % CI 0·78, 0·93, P=0·001), suggesting that the observed result could be due to a possible higher activity of lignans with low oestradiol levels.
In vitro and in vivo trials described different mechanisms by which polyphenols act in cancer prevention and treatment. So hydroxytyrosol may have antioxidants effects and thus protect from DNA damage; resveratrol may inhibit cell proliferation inducing the apoptosis mechanisms; curcumin may exert anti-inflammatory properties; and several polyphenols such as hydroxytyrosol, oleuropein, quercetin and resveratrol may inhibit angiogenesis and cell migration. There are some further mechanisms that may link polyphenol intake and BC risk and more specifically postmenopausal BC risk. Several flavonoids have a similar structure to natural oestrogens, can selectively bind to oestrogen receptor (ER) β and, thus, suppress cell proliferation. Hydroxycinnamic acids, the major contributors to total phenolic acid intake, can inhibit cell proliferation, modulate apoptosis and ERα expression, and have antioxidant activity( Reference Rocha, Monteiro and Teodoro36 ). Some polyphenols, as curcumin and olive oil lignans, have shown human epidermal growth factor receptor 2 (HER2) modulation capacity. Finally, flavonoids and resveratrol demonstrated the ability to inhibit aromatase activity. This inhibition decreases the peripheral conversion of androgens to oestrogens, a key mechanism for postmenopausal BC( Reference Gorzynik-Debicka, Przychodzen and Cappello5 , Reference Losada-Echeberría, Herranz-López and Micol7 , Reference Mocanu, Nagy and Szöllősi8 ).
We acknowledge that this work has some limitations. First, we are aware that our statistical power is compromised by the limited number of incident cases of BC. Our cohort is composed largely by young women and most of them are premenopausal, which reduces the number of incident BC cases. Second, and due to the small number of incident BC cases, we did not perform a sub-analysis by subtypes of BC cases. Third, dietary information was based on self-reported information. This might have led to some misclassification bias. Nevertheless, the FFQ had been previously validated( Reference Martin-Moreno, Boyle and Gorgojo13 ), and the non-differential misclassification would bias our results towards the null value. Fourth, polyphenol content may not be uniform for foods across brands and times. For example, polyphenol content may vary across types of chocolate and coffee over time. This might have led to a non-differential misclassification of the exposure which could have biased our results towards the null. Fifth, the absence of information regarding spices and herbs in the FFQ might have resulted in an underestimation of the polyphenol intake, as, although they are consumed in small amounts, they provide a huge number of polyphenols( Reference Pérez-Jiménez, Neveu and Vos37 ). Sixth, information on BC incidence also was self-reported. To confirm the accuracy of the information, we asked our participants to send a copy of their medical reports. We run our analyses separately, considering on the one hand those cases that could be confirmed with the medical reports; and on the other hand, all the cases including those that had no medical report confirmation by the day of the analysis. In spite of this, we might have missed some cases of BC. This fact might have reduced the sensitivity to detect incident cases of BC. However, the close follow-up of our participants and the periodic consultation of the National Death Index will have limited the number of missed BC cases. Finally, blood samples from our participants were not available. Therefore, we could not validate the estimated consumption of polyphenols in our cohort.
On the other hand, our study also shows some strengths. It is a dynamic cohort with more than 16 years of follow-up, with 91 % retention rate, and which includes actually 13 843 women. Also, we have exhaustive information about the diet of the participants, collected by the FFQ mentioned before. Regarding polyphenol content of foods, we used the Phenol-Explorer database (www.phenol-explorer.eu)( Reference Neveu, Perez-Jimenez and Vos15 ) to classify them in an accurate manner. Moreover, the FFQ has been previously validated and represents the main foods consumed by the study population( Reference De la Fuente-Arrillaga, Vázquez Ruiz and Bes-Rastrollo38 ). In previous studies, our group validated the FFQ to assess total polyphenol intake in both clinical and cross-sectional studies( Reference Medina-Remón, Barrionuevo-González and Zamora-Ros39 ). Moreover, we collected a wide array of potential confounders which have been included in the multivariable analyses, which reduces the room for residual confounding and other potential biases in our results.
Conclusion
In conclusion, in this Mediterranean cohort study, we observed no significant association between total polyphenol intake and overall BC risk although we found a statistically significant association between total polyphenol intake and the risk of developing a BC among postmenopausal women. Also, among postmenopausal women, we found an inverse association between phenolic acid intake and postmenopausal BC. This is one of the first works evaluating the role of total polyphenol intake and BC risk. Due to the large in vivo and in vitro evidence, more studies are needed to disentangle the association between polyphenol intake and the risk of overall BC and by subgroups.
Acknowledgements
The authors are indebted to the participants of the SUN study for their continued cooperation and participation. The authors are also grateful to the members of the Department of Nutrition of the Harvard School of Public Health (W. C. Willett, F. B. Hu and A. Ascherio) who helped us to design the SUN study. The authors also thank other members of the SUN group: A. Alonso, M. T. Barrio López, F. J. Basterra-Gortari, S. Benito Corchón, M. Bes-Rastrollo, J. J. Beunza, S. Carlos Chillerón, L. Carmona, S. Cervantes, J. de Irala Estévez, C. de la Fuente Arrillaga, P. A. de la Rosa, M. Delgado Rodríguez, C. L. Donat Vargas, M. Donázar, A. Fernández Montero, C. Galbete Ciáurriz, M. García López, A. Gea, E. Goñi Ochandorena, F. Guillén Grima, A. Hernández, F. Lahortiga, J. Llorca, C. López del Burgo, A. Marí Sanchís, A. Martí del Moral, N. Martín-Calvo, J. A. Martínez, R. D. Mendonça, J. M. Núñez-Córdoba, P. Pérez de Ciriza, A. M. Pimenta, R. Ramallal, A. Ruiz Zambrana, A. Rico Campà, M. Ruiz-Canela, D. Sánchez Adán, C. Sayón Orea, Z. Vázquez Ruiz and I. Zazpe García.
This work was supported by the Spanish Government-Instituto de Salud Carlos III and the European Regional Development Fund (FEDER) (RD 06/0045, CIBER-OBN, grants PI10/02658, PI10/02293, PI13/00615, PI14/01668, PI14/01798, PI14/01764, PI17/01795 and G03/140), the Navarra Regional Government (45/2011, 122/2014 and 41/2016) and the University of Navarra. A. R. N. is supported by the Asociación Española Contra el Cáncer (Spanish Association Against Cancer).
I. G. and E. T. wrote the draft of the manuscript; I. G., A. R.-N., R. S.-B. and E. T. were responsible for data analysis; M. A. M.-G. and E. T. were responsible for conception and design, data acquisition and interpretation. All coauthors revised the manuscript critically for important intellectual content and approved the final version to be published.
None of the other authors has conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518003811












