The intestine is a crucial interface for nutrient and non-nutrient substances and energy exchange between an animal and its environment; this part of the digestive system provides the first barrier against hazardous substances, such as carcinogens, dietary-derived mutagens and oxidants( Reference Cencic and Langerholc 1 ). The primary role of the intestine is to digest food and absorb nutrients that are subsequently transported to different tissues and organs via blood circulation for body growth. Moreover, the intestine can trigger signals to the central nervous system, thereby influencing the balance among internal environments, substances and energy. The integrated structures and normal functions of the intestine are directly related to the overall health status of the body, suggesting that maintenance of gut homoeostasis is important for animal growth and development. Intestinal homoeostasis is a state of dynamic equilibrium achieved through complex interactions between the intestinal mucosa, intestinal immune barrier, enteric microorganisms, intestinal nutrients and intestinal metabolites( Reference Maloy and Powrie 2 ). Gut homoeostasis plays a significant role in normal physiological functions, such as nutrient absorption, energy metabolism and reduction of intestinal infections; thus, imbalance can induce numerous diseases, including obesity, neurodevelopmental disorder and inflammatory bowel disease( Reference Wei, Yang and Rey 3 , Reference Hsiao, McBride and Hsien 4 ).
Nutrient-sensing receptors are present in the mammalian epithelium lining the intestine and can effectively detect and respond according to the state of the microbial environment, especially microbial and nutrient composition. The receptors regulate normal intestinal functions, such as absorption and secretion, according to the state of digestion and nutrient availability. The receptors also regulate gut permeability, integrity and immunity according to the structure of the intestinal flora or the organisation of the microbial population. One of the important nutrient-sensing receptors is the extracellular Ca-sensing receptor (CaSR).
CaSR, a well-conserved and ancient guanine nucleotide-binding protein (G protein)-coupled receptor (GPCR), maintains systemic extracellular Ca2+ homoeostasis by modulating parathyroid hormone secretion( Reference Lin, Chattopadhyay and Bai 5 , Reference Ba and Friedman 6 ). Notably, CaSR plays other crucial roles in the physiology and pathophysiology of cellular functions involved in the growth, development and health of organisms( Reference Ward, Magno and Walsh 7 – Reference Lopez-Fernandez, Schepelmann and Brennan 9 ). CaSR can activate various tissue-specific signalling pathways upon agonist or allosteric activator stimulation and thus play critical roles in various biological processes, such as hormone secretion, cell proliferation, cell differentiation, apoptosis, ion channel activity, cell membrane potential regulation and modulation of signalling molecules for gene and protein expression( Reference Lin, Chattopadhyay and Bai 5 , Reference Ba and Friedman 6 , Reference Tennakoon, Aggarwal and Kállay 10 , Reference Brown and MacLeod 11 ). Abnormal CaSR expression and/or activity are closely related not only to disorders of the parathyroid gland but also to other diseases, such as diarrhoea, colitis and neoplasia( Reference Geibel, Sritharan and Geibel 12 – Reference Fraebel, Gonzalez-Peralta and Maximos 15 ). Therefore, CaSR is important in the maintenance of animal health. Elucidating the effects of CaSR on the modulation of intestinal homoeostasis offers great theoretical and practical significance. In this review, we summarised the roles of CaSR in intestinal homoeostasis and partially offered experimental data for further studies on the effects and underlying mechanisms of CaSR.
Overview of calcium-sensing receptor
Origin of calcium-sensing receptor
Brown et al.( Reference Brown, Gambag and Riccardi 16 ) first cloned approximately 5·4 kb-long transmembrane receptor gene from a bovine parathyroid gland, and the receptor utilised Ca2+ as a physiological ligand, consequently naming the receptor CaSR in 1993. CaSR is capable of sensing minor fluctuations in extracellular Ca2+ concentration (approximately 200 µmol/l) and can accurately control its concentration in serum. CaSR mainly regulates parathyroid hormone secretion and Ca2+ homoeostasis.
Structural characteristics of calcium-sensing receptor
CaSR is an exceptionally large protein that contains 1078 amino acids and has three intracellular loops and a large carboxyl terminal domain. CaSR is composed of four major parts with regard to its structure, namely the extracellular domain (ECD), a cysteine-rich domain linking the ECD to the first transmembrane helix, a seven transmembrane domain and intercellular C-terminal domain( Reference Garrett, Capuano and Hammerland 17 – Reference Bai, Quinn and Trivedi 20 ), as shown in Fig. 1. ECD is a large domain consisting of approximately 600 amino acids with an extracellular N terminal domain, equipped with the characteristic topology of several GPCR – a venus flytrap structure( Reference Hofer and Brown 19 ). The cysteine-rich domain linking the ECD to the transmembrane domain plays an important role in conducting signals to the seven transmembrane domain( Reference Hofer and Brown 19 ). ECD comprises a hydrophilic amino terminal group (–NH2), which enables the detection of some nutrients. The venus flytrap structure is located outside of the cell and detects several nutrients, including amino acids, peptides and polyamines( Reference Tang, Cheng and Sun 21 ).
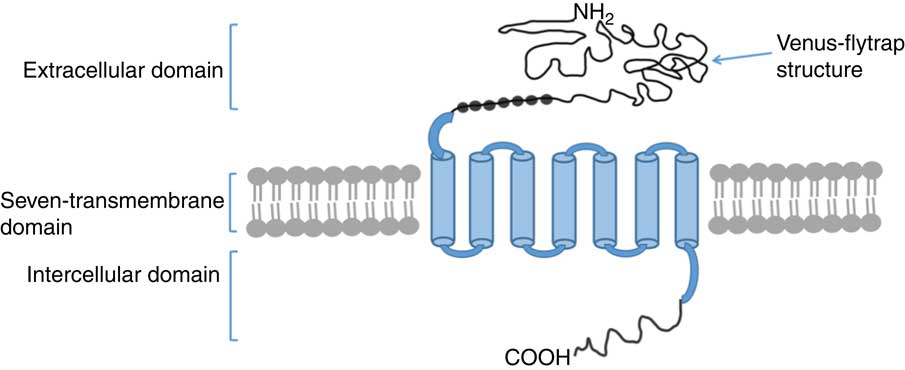
Fig. 1 Structure model of monomer of calcium-sensing receptor(
Reference Mine and Zhang
13
,
Reference Brennan, Thiem and Roth
14
,
Reference Brown, Gambag and Riccardi
16
–
Reference Zhang, Miller and Brown
18
). The extracellular domain contains venus flytrap structure which serves as the major ligand for agonistic interaction. ![]() , Individual cysteine residues that involve the cysteine-rich domain. The carboxyl terminous is extended into the cytoplasm.
, Individual cysteine residues that involve the cysteine-rich domain. The carboxyl terminous is extended into the cytoplasm.
Distribution of calcium-sensing receptor
CaSR is widely distributed into the tissues of the animal or human body. CaSR is not only highly expressed in the kidney, bone tissues, thyroid, parathyroid and stomach, but also in the pancreas, marrow, breasts, pituitary gland, liver and vascular smooth muscle( Reference Wonneberger, Scofield and Wangemann 22 – Reference Riccardi, Park and Lee 25 ). CaSR can also be found in the intestine( Reference Geibel and Hebert 26 – Reference Aggarwal, Prinz-Wohlgenannt and Tennakoon 29 ). CaSR is expressed in the epithelial cells of intestine and enteric nervous system( Reference Chattopadhyay, Cheng and Rogers 30 – Reference Cheng 32 ). In small intestine, CaSR is expressed in both apical and basolateral membranes of villus cells( Reference Chattopadhyay, Cheng and Rogers 33 ). CaSR is identified in the submucosa and muscularis of the ileum and jejunum( Reference Chattopadhyay, Cheng and Rogers 30 , Reference Gama, Baxendale-Cox and Breitwieser 34 ). In the large intestine, CaSR is primarily expressed in the basal, lateral and apical membranes of epithelial cells located in the crypt and villus and in the basal endocrine cells of colonic crypt( Reference Hebert, Cheng and Geibel 35 – Reference Fetahu, Höbaus and Aggarwal 38 ).
Roles of calcium-sensing receptor in gut homoeostasis
The intestinal mucosa enables interactions between the internal and external environments and exhibits vital functions in digestion, absorption, secretion and activity against toxic substances. Normal digestion and absorption require intact structures of the intestinal mucosal epithelia( Reference Flint, Scott and Louis 39 , Reference Peterson and Artis 40 ). Many nutrients can bind to the site of CaSR, activating signalling pathways and regulating animal growth, development and health status. CaSR can maintain gut homoeostasis by accelerating development and maturation, enhance the immune system and preserve barrier functions.
Calcium-sensing receptor and intestinal development
CaSR facilitates epithelial cell proliferation and differentiation in the intestine. The intestine is the primary site for digestion and nutrient absorption. Intestinal cells in the mucosal barrier undergo continuous turnover. Cell proliferation and differentiation occur when cells migrate from the base of the intestinal crypt to its apex, and this migration is a determinant of intestinal growth and development( Reference Cummins and Thompson 41 ). CaSR influences intestinal development and growth by modulating intestinal cell proliferation and differentiation. Rey et al.( Reference Rey, Chang and Bikiel 36 ) first observed that epithelial CaSR deficiency in vivo results in changes in crypt structure and expansion of proliferative zones. Researchers assessed cell differentiation ex vivo by measuring [3H]thymidine incorporation into cellular DNA and found that CaSR activation causes Caco-2 cell proliferation( Reference Aggarwal, Prinz-Wohlgenannt and Tennakoon 29 , Reference Kállay, Kifor and Chattopadhyay 42 ). CaSR expression in mice fed with a diet high in vitamin D increases colonic apoptosis( Reference Aggarwal, Höbaus and Tennakoon 43 ), suggesting a molecular link supporting positive cross-talk between the vitamin D system and CaSR. Moreover, cell apoptosis may be facilitated by CaSR and bone morphogenetic protein 2 (BMP-2), which can enhance apoptosis in colonic cell lines( Reference Peiris, Pacheco and Spencer 44 ). In this study, the activation of CaSR by agonists increased BMP-2 gene and protein expression levels in human colonic myofibroblasts. However, CaSR knockdown by small interfering RNA or transient transfection with a dominant negative CaSR mutant suppressed BMP-2 synthesis and secretion, suggesting that CaSR promotes cell apoptosis. Apart from cell apoptosis, intestinal development and growth are intimately related to its morphological structures. In mice, poly-l-lysine feeding prevents the shortening of colon length induced by dextran sulphate sodium salt, although this effect is abrogated after the injection of a CaSR inhibitor (NPS-2143)( Reference Mine and Zhang 13 ). Furthermore, spermine serves as an important CaSR agonist. Spermine supplementation effectively enhances villus height, villus width and rat jejunum surface area( Reference Cao, Liu and Fang 45 ). These observations suggest that CaSR activation by agonists promotes intestinal development.
Underlying mechanisms of intestinal development induced by calcium-sensing receptor
There is little information about signalling pathways activated by CaSR that regulate intestinal development. One study revealed the presence of CaSR on colonic epithelial cells( Reference Aggarwal, Höbaus and Tennakoon 43 ). In mice, the conditional knocking out of CaSR reduced β-catenin phosphorylation in the colon, whereas CaSR stimulation increased phosphorylation of β-catenin in a cell line derived from normal colon mucosal epithelium. Importantly, β-catenin primarily functions in the modulation of the canonical Wnt/β-catenin signalling pathway, which is essential to intestinal development and enhances epithelial proliferation and differentiation( Reference Rey, Chang and Bikiel 36 , Reference Clevers 46 – Reference Aggarwal, Prinz-Wohlgenannt and Gröscel 48 ). Thus, CaSR activation mediates gut cell proliferation and differentiation probably via β-catenin signalling and affects intestinal development and growth.
Meanwhile, CaSR regulates multitudinous downstream signalling pathways in other cells or tissues. CaSR stimulation activates G protein-mediated phospholipase C (PLC) and phosphatidylinositol bisophosphate, causing the release of inositol triphosphate (IP3) and diacylglycerol (DAG)( Reference Buchan, Squires and Ring 49 ); thereafter, CaSR induces IP3-mediated intracellular Ca2+ release because IP3 can diffuse and bind to the endoplasmic reticulum while binding to its specific receptors( Reference Hofer and Brown 19 ). Subsequently, an increase in intracellular DAG or Ca2+ concentration stimulates protein kinase C, triggering mitogen-activated protein kinase (MAPK)-associated signal transduction( Reference Schönwasser, Marais and Marshall 50 ). CaSR activation also stimulates MAPK-dependent signalling proteins, such as c-Jun N-terminal kinases (JNK), P38 MAPK and extracellular signal-regulated kinase 1/2( Reference Tfelt-Hansen, MacLeod and Chattopadhyay 51 ). MAPK-dependent signal transduction strongly improves activator protein 1 (AP-1) expression. AP-1 is a regulatory factor for cell proliferation and plays vital roles in tissue development. These findings imply that CaSR participates in cell proliferation and differentiation through cascades of intracellular signalling transduction (Fig. 2).
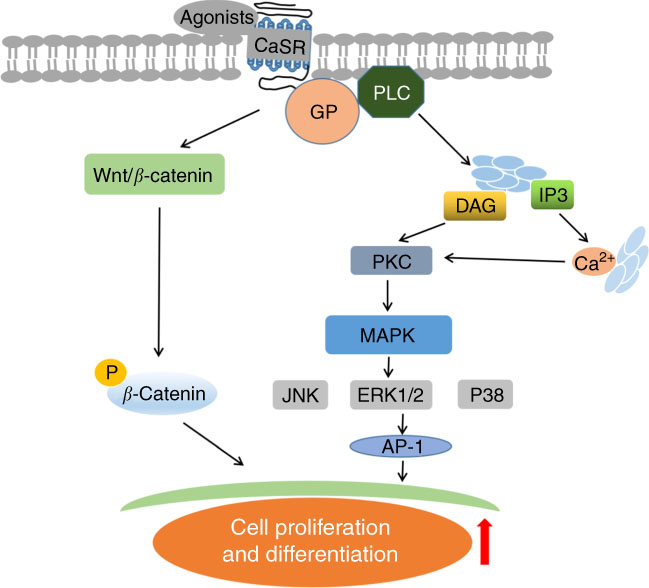
Fig. 2 Schematic diagram of underlying mechanisms of intestinal development upon calcium-sensing receptor (CaSR) activation. PLC, phospholipase C; IP3, inositol triphosphate; DAG, diacylglycerol; PKC, protein kinase C; MAPK, mitogen-activated protein kinase; JNK, c-Jun N-terminal kinases; ERK1/2, extracellular signal-regulated kinase 1/2; GP, G proteins; AP-1, activator protein 1.
Calcium-sensing receptor and intestinal hormone secretion
The intestine is an essential tissue for the survival and growth of mammals, owing to its primary function in digestion and nutrient absorption. Different cell types are located in the mucosa lining the intestine. These cells gradually differentiate after migrating out of the crypt, which subsequently synthesise and secrete enzymes and hormones. Intestinal hormones promote nutrient digestion and absorption, elevating nutrient availability and reducing the risk of diseases( Reference Lund, Vilsboll and Bagger 52 ) and are thus closely associated with gut homoeostasis. CaSR can modulate hormone secretion( Reference Macleod 53 ). Understanding changes in hormone levels under CaSR activation and/or inhibition is fairly meaningful. Liou et al.( Reference Liou, Sei and Zhao 31 ) detected the CaSR from enriched duodenal I cells that can be allosterically stimulated by a known cholecystokinin (CCK) secretagogue, l-phenylalanine. Normal mice with retained CaSR allele or double-CaSR-knockout mice exhibit distinct CCK secretion. CCK can lead to the release of digestive enzymes and bile from the pancreas and gallbladder. Native I cells originating from normal mice with retained CaSR allele supplemented with appropriate l-phenylalanine showed enhanced CCK secretion. In contrast, cells derived from double-CaSR-knockout mice did not secrete CCK, suggesting that CaSR mediates l-amino acid-induced CCK secretion. In another experiment involving male rats, the perfused loops of the small intestine were used for the examination of CaSR-mediated gut peptide secretion( Reference Mace, Schindler and Patel 54 ). In this study, gluco-insulinotropic peptide (GIP), glucagon-like peptide-1 (GLP-1) and peptide tyrosine secretion from K and L cell of the small intestine were stimulated by l-amino acids, such as glutamine, phenylalanine, tryptophan, asparagine and arginine. However, these effects were suppressed by CaSR antagonist (i.e. Calhex 231) pretreatment but were induced again after the application of a specific CaSR agonist (i.e. NPS R-685). Notably, the dominating role of GLP-1 and GIP is to augment glucose-induced insulin secretion from β cells( Reference Tang, Cheng and Sun 21 ), suggesting that CaSR functions on nutrient utilisation and energy homoeostasis. As described above, CaSR expression in small intestinal epithelial cells possibly plays a significant role in sensing nutrients. Nevertheless, the detailed mechanisms of CaSR in modulating intestinal hormones secretion need further investigation.
Roles of calcium-sensing receptor in intestinal barrier
Intestinal barrier function involves the intestinal epithelium, which plays an important role in separating matters by the lumen and preventing the invasion of pathogenic substances. Under natural physiological conditions, physical, chemical, immunological and microbial barriers of the gut work together to maintain intestinal barrier function. The effects of CaSR on intestinal barrier function mainly manifest in physical, immunological and microbial barriers of the gut.
Calcium-sensing receptor and intestinal physical barrier
The epithelial monolayer of the intestine primarily serves as the physical barrier. Intestinal physical barrier refers to intact epithelium structure, which contains junctional complexes, such as tight junctions, adherens junctions, desmosomes and gap junctions( Reference Owen, Cheng and Ge 55 ). Furthermore, this tissue enables the selective transport of solutes, essential nutrients and water via two routes: transcellular and paracellular pathways. The selectivity of intestinal epithelium depends on the permeability of paracellular pathway achieved through the intercellular space between adjacent cells; this permeability can be determined using fluorescein isothiocyanate (FITC) dextran( Reference Stenman, Holma and Gylling 56 ). Recently, an experiment using CaSR knockout mice showed increased passive transport of FITC-conjugated dextran in the colon( Reference Cheng, Lightfoot and Yang 57 ), suggesting that CaSR effectively maintains intestinal permeability by affecting the paracellular transport pathway. In addition, paracellular pathway is also associated with the state of the tight junction, which involves complex interactions between transmembrane proteins and ion transporter-associated enzymes, such as myosin light-chain kinase-1 (MLCK1). Transmembrane proteins, especially occludin and claudin, are important components of intestinal tight junctions( Reference Tsukita and Furuse 58 ). MLCK1 is an enzyme that controls gut epithelial permeability, and its enhanced expression causes destruction of intestinal tight junctions( Reference Graham, Wang and Clayburgh 59 ). Consistent with increased intestinal permeability, real-time PCR showed decreased claudin two mRNA level and improved MLCK1 gene expression in the colon of CaSR−/− mice( Reference Cheng, Lightfoot and Yang 57 ). Notably, the functional state of intestinal tight junctions partially relies on transepithelial resistance (TEER)( Reference Schmitz, Fromm and Bentzel 60 ). CaSR−/− mice also demonstrate markedly reduced TEER and increased transepithelial conductance( Reference Cheng, Lightfoot and Yang 57 ). According to the above-mentioned results, CaSR can modulate the intestinal physical barrier.
Calcium-sensing receptor and intestinal microbial barrier
In addition to handling many dietary nutrients, the intestine harbours multiple microorganisms, especially in the colon. These microorganisms effectively prevent exogenous toxicants from entering the body. During long-term evolution process, the gut microbiota and innate intestinal structures work together to form a micro-ecosystem. Under normal conditions, the living function of an animal is maintained by a dynamic balance between the microbiota, host and environment. Consequently, the functions and health status of the intestine are closely related to its microbial composition. An increasing number of studies have illuminated the mutual relationship and bidirectional interactions between gut microbiota and nutrition through the characterisation of gut microbiota composition and function by 16S ribosomal DNA (rDNA) high-throughput sequencing( Reference Maukonen and Saarela 61 ). Many mechanisms were proposed to explain these interactions. CaSR is also expressed on immune cells and contributes to their activation; thus, there is also an interaction between the intestinal epithelial cells, the small molecules produced by the microbiome and the organismal immune cells targeted to the area. Based on these findings, we speculated that CaSR participates in the regulation of intestinal microbial barrier as a nutrient sensor by affecting microbiota composition. A previous study analysed the microbiota composition via 16S rDNA-sequencing technique in steady-state CaSR−/− and wild-type mice( Reference Cheng, Lightfoot and Yang 57 ). Interestingly, no significant difference between diversity and the overall richness of the two gut microbial communities was found. Nevertheless, the results of comprehensive analyses demonstrate that epithelial CaSR deficiency alters bacterial composition. At the phylum level, an outgrowth of Deferribacteraceae was observed, which was thought to be intimately associated with inflammatory responses of the colon in Citrobacter rodentium-infected mice – a model of bacterial-mediated colitis( Reference Cheng, Lightfoot and Yang 57 ). Knockout CaSR mice exhibit a marked decrease in beneficial lactobacilli and Clostridium ( Reference Cheng, Lightfoot and Yang 57 ). Moreover, deficiency in epithelial CaSR changes the relative abundance and distribution of the Gram-positive organism Clostridium coccoides, finding its depletion in the lumen and its enrichment in the subepithelium( Reference Cheng, Lightfoot and Yang 57 ). In agreement with increased bacterial translocation and dissemination in host tissues, epithelial CaSR deficiency significantly reduces Reg3β and Reg3γ gene expressions of the distal colon( Reference Cheng, Lightfoot and Yang 57 ), which plays a vital role in encoding secreted C-type lectins that bind and protect against translocation and dissemination of Gram-negative and Gram-positive bacteria( Reference van Ampting, Loonen and Schonewille 62 , Reference Cash, Whitham and Behrendt 63 ), respectively. In the light of these observations, CaSR can regulate the intestinal microbial barrier by influencing the magnitude and distribution of microbiota. However, little is known about the detailed mechanisms of this action, and further studies are thus required to address such problems.
Calcium-sensing receptor and intestinal immune barrier
Effects of calcium-sensing receptor on modulating gut immune barrier function
The mammalian intestine is not only the largest digestive organ but also the largest immune organ. Numerous chemical substances, including chemokines, digestive secretions, inflammatory mediators, cytokines and antimicrobial peptides, participate in intestinal barrier function. The colon exhibits a steady state of inflammation; the magnitude of which is mainly modulated by the immune barrier provided by the intestine. Therefore, we conjectured that the dysbiosis of intestinal immune barrier causes pathogenic inflammatory immune responses, and this hypothesis was verified by gene array analyses of the distal colon of wild-type and knockout CaSR mice( Reference Cheng, Lightfoot and Yang 57 ). This study revealed that CaSR−/− mice partly demonstrate the relationship between the development and maturation of intestinal immune system and CaSR. The enhanced number of B cells and expression levels of IgA were observed in the colon of CaSR−/− mice( Reference Cheng, Lightfoot and Yang 57 ). In the mesenteric lymph nodes, epithelial CaSR deficiency significantly increases the number of FoxP3+ regulatory T cells( Reference Cheng, Lightfoot and Yang 57 ) which is a subgroup of CD4+ T helper cell that play a central role in intestinal homoeostasis. These results suggest that CaSR locally maintains the function of intestinal immune barrier by affecting intestinal immune cells. Moreover, CaSR may be involved in the regulation of inflammatory responses closely correlated with immune status. In vitro studies reveal that epithelial CaSR inhibition increases IL-8 secretion and IL-6 and TNF-α protein expression in cultured intestinal epithelial cells( Reference Mine and Zhang 13 , Reference Mine and Zhang 64 ). However, epithelial CaSR activation can decrease TNF-α secretion in colonic myofibroblasts( Reference Kelly, Lungchukiet and MacLeod 65 ). Consistently, an in vivo experiment demonstrated that CaSR−/− mice showed a dramatic enhancement in the mRNA expression of numerous pro-inflammatory cytokines of the colon, such as IL-17, IL-6, IL-1β, interferon γ and nitric oxide synthase( Reference Cheng, Lightfoot and Yang 57 ). Glutathione analogue-induced CaSR stimulation effectively decreases inflammatory cytokine gene expression and increases anti-inflammatory cytokine gene expression, including IL-10 in mice colon( Reference Zhang, Kovacs-Nolan and Kodera 66 ). Furthermore, CaSR-deficient mice exhibited more rigorous colitis induced by dextran sodium sulphate with delayed recovery relative to their littermate counterparts( Reference Cheng, Lightfoot and Yang 57 ), as evidenced by the increased weight loss, enhanced stool consistency and incomplete morphology. This result suggests that intestinal CaSR plays an important role in the alleviation of inflammatory disorders. Collectively, CaSR preserves intestinal immune barrier by participating in the modulation of intestinal immune cell maturation and inflammatory immune responses.
Underlying mechanisms of intestinal immune barrier upon calcium-sensing receptor
CaSR agonists recruit various functional signalling proteins and control diverse signalling networks, including the interference of TNF-α-stimulated signalling pathways. As a family of such functional signalling proteins, β-arrestin family comprises multifunctional scaffold proteins capable of binding receptors phosphorylated by GPCR kinases. β-Arrestin2 is involved in CaSR-mediated anti-inflammatory effects( Reference Kelly, Lungchukiet and MacLeod 65 , Reference Gao, Sun and Wu 67 ). In β-arrestin2-deficient Caco-2 and HT-29 cells, CaSR-induced anti-inflammatory effects by GSH analogues or l-amino acid are abolished, thereby enhancing IL-8 levels. This finding implies that β-arrestin2 expression is required for CaSR-mediated anti-inflammatory activity. Moreover, the interaction of β-arrestin2 with various downstream signalling components in inflammatory immune responses, such as those involving JNK and NF-κB inhibitor protein (IκB), is important( Reference DeWire, Ahn and Lefkowitz 68 , Reference Witherow, Garrison and Miller 69 ). β-Arrestin2 expression in intestinal epithelial cells is required for JNK and IκB phosphorylation, as demonstrated by the reduced TNF-α-induced JNK and IκB phosphorylation after CaSR activation( Reference Zhang, Kovacs-Nolan and Kodera 66 ). This result suggests that the underlying mechanisms of CaSR-mediated anti-inflammation may involve the interaction of β-arrestin2 with upstream signalling proteins in the TNF-α signalling pathway. TNF-α receptor (TNFR) is important in regulating inflammation. Upon the binding of TNF-α to TNFR, a trimeric complex is generated by interacting with transforming growth factor (TGF)-β-activated kinase 1 (TAK1), TAK1-binding protein (TAB)1 and TAB2 in the cytoplasm when TNFR-associated factors disconnect from the receptor, mediating the activation of NF-κB and AP-1 signalling pathways and resulting in an inflammatory response( Reference Liu, Busby and Molkentin 70 , Reference Ninomiya-Tsuji, Kishimoto and Hiyama 71 ). The studies demonstrated that GSH analogues and l-amino acid can cause an association between β-arrestin2 and CaSR, resulting in the interaction of β-arrestin2 with TAB1 and decreased TNF-α-induced TAK1–TAB1 complex generation in intestinal epithelial cells( Reference Mine and Zhang 64 , Reference Zhang, Kovacs-Nolan and Kodera 66 ). Similarly, CaSR activation fails to suppress TNF-α-mediated TAB1–TAK1 association in β-arrestin2-knockdown cells. These findings suggest that CaSR stimulation can maintain the intestinal immune barrier through the combination of β-arrestin2 with TAB1 to prevent the activation of pro-inflammation-dependent inflammatory signalling pathways.
In addition, the presence of β-arrestin2 in the modulation of Wnt/Ca2+ and Wnt/receptor tyrosine kinase like orphan receptor 2 (Ror2) pathways is essential. The activation of Wnt/Ca2+/calmodulin-dependent protein kinase II by Wnt5a accelerates NF-κB stimulation, which evokes endothelial inflammation( Reference Kim, Kim and Kim 72 ). This effect suggests that Wnt secretion and signalling play a central role in the modulation of innate and adaptive immune responses. To examine this novel role, researchers demonstrated the relationship between CaSR-mediated anti-inflammatory effects and Wnt5a/Ror2 engagement in colonic myofibroblasts and intestinal epithelial barriers challenged by lipopolysaccharide( Reference Kelly, Lungchukiet and MacLeod 65 ). In the experiment, CaSR activation can inhibit TNF-α secretion in colonic myofibroblasts, enhance Ror2 expression in intestinal epithelia and induce Wnt5a secretion and expression in colonic myofibroblasts. The interaction of CaSR-mediated Wnt5a with Ror2 significantly decreased TNFR1 protein expression on intestinal epithelia. Decreased TNFR1 can reduce barrier damage by blocking TNF-α-dependent signalling pathways. Likewise, in vivo studies showed results as described above( Reference Turki, Kelly and Pacheco 73 , Reference MacLeod 74 ). In summary, these findings imply that CaSR activation inhibits TNFR1 expression on intestinal epithelia via Wnt5a/Ror2 engagement, subsequently preserving the gut immune barrier (Fig. 3).
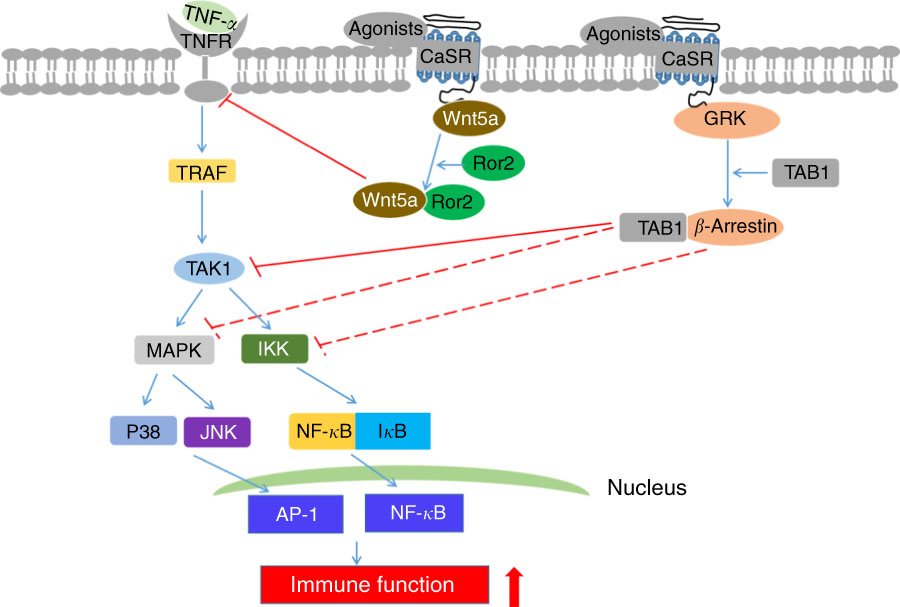
Fig. 3 Schematic diagram of underlying mechanisms of intestinal immune barrier upon calcium-sensing receptor (CaSR) activation. ![]() and
and ![]() T shapes indicate direct and indirect inhibitory effects, respectively. TNFR, TNF-α receptor; TRAF, TNFR-associated factor; IκB, NF-κB inhibitor protein; TAK1, transforming growth factor-β-activated kinase 1; GRK, G-protein-coupled cell surface receptor kinase; TAB1, TAK1 binding protein; AP-1, activator protein 1; MAPK, mitogen-activated protein kinase; IKK, IκB kinase; JNK, c-Jun N-terminal kinases; Ror2, receptor tyrosine kinase like orphan receptor 2.
T shapes indicate direct and indirect inhibitory effects, respectively. TNFR, TNF-α receptor; TRAF, TNFR-associated factor; IκB, NF-κB inhibitor protein; TAK1, transforming growth factor-β-activated kinase 1; GRK, G-protein-coupled cell surface receptor kinase; TAB1, TAK1 binding protein; AP-1, activator protein 1; MAPK, mitogen-activated protein kinase; IKK, IκB kinase; JNK, c-Jun N-terminal kinases; Ror2, receptor tyrosine kinase like orphan receptor 2.
Calcium-sensing receptor and intestinal secretory diarrhoeas
Calcium-sensing receptor mitigates intestinal secretory diarrhoeas
Both the submucosal Meissner’s plexus and myenteric Auerbach’s plexus cause diarrhoea when disturbed. In humans and rodents, at least 50 % of the fluid secreted in cholera, rotavirus and other forms of secretory diarrhoea is caused by the activation of the enteric nervous system(
Reference Burleigh and Borman
75
–
Reference Lundgren, Peregrin and Persson
78
). These fluids are also present in patients with irritable bowel syndrome(
Reference Wood
79
), inflammatory bowel disease(
Reference Margolis, Stevanovic and Karamooz
80
) and intestinal allergies. Colonic epithelial crypts provide a model that supports the entry of intestine-secreted fluid, and this action is mainly driven by transepithelial secretion of anions, such as Cl–. In a definitive study, the role of colonic CaSR in the regulation of intestinal fluid secretion was established through the comparison of secretagogue-stimulated Cl– responses with those of CaSR agonist Ca2+ and R568 in colonic crypts-deficient CaSR mice with wild-type controls(
Reference Geibel, Sritharan and Geibel
12
). Two effective secretagogs, namely forskolin and cholera toxin, promote basolateral membrane Cl– entry into colonocytes by detecting N-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide fluorescence responses sensitive to Cl–; moreover, this effect is abrogated by CaSR stimulated from either the mucosal or serosal side by Ca2+ and R568(
Reference Geibel, Sritharan and Geibel
12
). Apart from Cl– secretion, increased
![]() $\rm HCO_{3}^{{\,\minus}} $
secretion caused by secretagogue also contributes to intestinal secretory diarrhoea. Thus, the suppression of
$\rm HCO_{3}^{{\,\minus}} $
secretion caused by secretagogue also contributes to intestinal secretory diarrhoea. Thus, the suppression of
![]() $\rm HCO_{3}^{{\,\minus}} $
transepithelial secretion by CaSR activation was investigated(
Reference Tang, Peng and Liu
81
). Results demonstrated that R568-mediated CaSR stimulation inhibits forskolin-induced
$\rm HCO_{3}^{{\,\minus}} $
transepithelial secretion by CaSR activation was investigated(
Reference Tang, Peng and Liu
81
). Results demonstrated that R568-mediated CaSR stimulation inhibits forskolin-induced
![]() $\rm HCO_{3}^{{\,\minus}} $
secretion in colonic apical and basolateral membranes of wild-type mice but not in CaSR–/– mice. Notably, CaSR can also be found in absorbing villus/surface cells, speculating important roles in regulating intestinal ion absorption. To address this hypothesis, the effects of CaSR activation on ion absorption in colonic crypts or mucosa derived from rats and mice are determined(
Reference Geibel, Sritharan and Geibel
12
). CaSR stimulation enhances Na+-dependent proton extrusion rate of colonocytes after increasing basolateral bath Ca2+ concentration or adding R568 in the presence of forskolin, hinting that CaSR increases Na+ absorption. Importantly, the absorption efficiency of water and some ions, such as Na+ and Cl–, can be regulated by SCFA absorption, which is conducive for conserving fluid and electrolytes. The roles of CaSR in Na+ absorption and Cl– secretion lead to a hypothesis that CaSR stimulation also plays a crucial role in modulating SCFA absorption. Thus, an experiment using Ussing chamber-pH stat technique was performed(
Reference Tang, Peng and Liu
81
). As expected, R568-mediated CaSR activation improves SCFA absorption in the colon of wild-type mice but not in intestinal epithelium-deficient CaSR mice. In summary, these studies reported that CaSR contributes to the amelioration of intestinal secretory diarrhoeas by blocking intestinal net fluid secretion and enhancing solute absorption.
$\rm HCO_{3}^{{\,\minus}} $
secretion in colonic apical and basolateral membranes of wild-type mice but not in CaSR–/– mice. Notably, CaSR can also be found in absorbing villus/surface cells, speculating important roles in regulating intestinal ion absorption. To address this hypothesis, the effects of CaSR activation on ion absorption in colonic crypts or mucosa derived from rats and mice are determined(
Reference Geibel, Sritharan and Geibel
12
). CaSR stimulation enhances Na+-dependent proton extrusion rate of colonocytes after increasing basolateral bath Ca2+ concentration or adding R568 in the presence of forskolin, hinting that CaSR increases Na+ absorption. Importantly, the absorption efficiency of water and some ions, such as Na+ and Cl–, can be regulated by SCFA absorption, which is conducive for conserving fluid and electrolytes. The roles of CaSR in Na+ absorption and Cl– secretion lead to a hypothesis that CaSR stimulation also plays a crucial role in modulating SCFA absorption. Thus, an experiment using Ussing chamber-pH stat technique was performed(
Reference Tang, Peng and Liu
81
). As expected, R568-mediated CaSR activation improves SCFA absorption in the colon of wild-type mice but not in intestinal epithelium-deficient CaSR mice. In summary, these studies reported that CaSR contributes to the amelioration of intestinal secretory diarrhoeas by blocking intestinal net fluid secretion and enhancing solute absorption.
Underlying mechanisms of reduced intestinal secretory diarrhoeas upon calcium-sensing receptor
Many factors may be involved in the underlying mechanisms of secretory diarrhoea, and cyclic nucleotide emerges as a particularly important one. Phosphodiesterase (PDE) is an enzyme that degrades cyclic nucleotides in cells( Reference Beavo 82 ). CaSR-mediated increase in cyclic nucleotide degradation and decrease in forskolin-stimulated fluid secretion in the colon are maintained when intestinal cells were pretreated with isoform-nonspecific PDE inhibitor 3-isobutyl-1-methylxanthine( Reference Geibel, Sritharan and Geibel 12 ). In addition, PLC can activate PDE1 activity and subsequently reverse CaSR-induced increased cyclic nucleotide degradation( Reference Geibel, Sritharan and Geibel 12 , Reference Beavo 82 ). Moreover, CaSR activation induced by Ca2+ or R-568 stimulates PLC activity, which generates IP3; moreover, IP3 causes Ca2+ release in thapsigargin-sensitive stores. CaSR abrogates secretagogue-induced increase in fluid secretion by improving cyclic nucleotides destruction( Reference Geibel, Sritharan and Geibel 12 ). These observations suggest that CaSR can partly alleviate intestinal secretory diarrhoeas via PLC–PDE pathway. Fluid secretion into the lumen of colonic crypts largely depends on the role of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels( Reference Kunzelmann and Mall 83 ). Secretagogue-induced improvements in cellular accumulation of cyclic adenosine monophosphate facilitate protein kinase A phosphorylation processes that drive the translocation of stimulated CFTR channels to the luminal plasma membrane, resulting in Cl– secretion( Reference Kunzelmann and Mall 83 ). Interestingly, the bumetanide-sensitive Na-K-2Cl cotransporter (NKCC1) also enables Cl– entry into the lumen of colonic crypts, inducing continuous Cl– secretion and fluid flow( Reference Beavo 82 ). NKCC1 is inhibited after CaSR activation by Ca2+ or R-568, subsequently mitigating secretory diarrhoeas( Reference Geibel, Sritharan and Geibel 12 ). Furthermore, parallel Na–H exchanger (NHE) regulates the activities of the major components of fluid absorption. Cyclic nucleotides contribute in the suppression of Na+-dependent fluid absorption by inhibiting NHE activity( Reference Yun, Oh and Zizak 84 ). Increasing basolateral bath Ca2+ concentration or adding R-568 stimulates CaSR, thereby markedly improving NHE activity up to 8-fold( Reference Geibel, Sritharan and Geibel 12 ). This result implies that CaSR reduces secretory diarrhoeas by regulating NHE signalling and partly mitigates intestinal secretory diarrhoeas via three mechanisms, namely the PLC–PDE pathway, NKCC1 signalling and NHE signalling (Fig. 4).

Fig. 4 Schematic diagram of underlying mechanisms of reduced intestinal secretory diarrhoeas upon calcium-sensing receptor (CaSR) activation. ![]() T shapes indicate direct inhibitory effect. PLC, phospholipase C; GP, G proteins; PDE, phosphodiesterase; IP3, inositol triphosphate; NKCC1, Na-K-2Cl cotransporter; NHE, Na–H exchanger.
T shapes indicate direct inhibitory effect. PLC, phospholipase C; GP, G proteins; PDE, phosphodiesterase; IP3, inositol triphosphate; NKCC1, Na-K-2Cl cotransporter; NHE, Na–H exchanger.
Calcium-sensing receptor activation and nutritional regulation
Similar to various other cell-surface receptors, CaSR can evoke a complex array of biological effects only when it combines with extracellular ligands. Ca2+ is the staple ligand for CaSR but is only one of numerous activators of this interesting receptor. Accumulating evidence proves that various nutrients, including amino acids, peptides, polyamines, glutathione analogues and alkaline polysaccharides, can be used to activate CaSR due to its unique structure, thereby implying that nutritional regulation may be developed to support CaSR-mediated maintenance of intestinal homoeostasis (Table 1).
Table 1 Nutritional species for calcium-sensing receptor activation regulation

Conclusion and future perspectives
Intestinal epithelium CaSR participates in the maintenance of intestinal homoeostasis, as evidenced by increased intestinal cell proliferation and differentiation, increased cell apoptosis, changed intestinal morphology, improved hormone secretion, recovered physical barrier, enhanced immune barrier, reinforced microbial compositions and alleviated secretory diarrhoeas, using in vitro, ex vivo or in vivo models. Furthermore, evidence supports the underlying mechanisms of CaSR which involve intestinal cell proliferation and differentiation, immune homoeostasis and secretory diarrhoeas. However, many questions associated with intestinal homoeostasis remain unsolved and require further studies. For example, whether CaSR improves the length, weight and digestive enzymes activities of the intestine for the acceleration of intestinal development, and whether it affects intestinal antioxidant status and metabolism for intestinal homoeostasis remain unknown. The role of CaSR in intestinal damage and repair, and the specific molecular mechanism of CaSR in the maintenance of intestinal physical and microbial barriers are unresolved. CaSR was identified as a novel and promising therapeutic target for the treatment of various gut diseases such as inflammatory bowel disease. A fundamental challenge for future studies is the exploration of the roles of CaSR in the intestine and the exact mechanisms of CaSR-mediated intestinal health from the view of nutriology. This research may provide primary experimental data of the supporting role of CaSR in the resistance of animals against diseases.
Acknowledgements
The present study was financially supported by the Program for Discipline Construction in Sichuan Agricultural University (no. 03570126).
The authors’ contributions were as follows: G. L. and W. C. contributed to the preparation of the manuscript, figures and editing of the manuscript. G. J., H. Z., X. C. and J. W. were involved in the preparation of the manuscript.
The authors declare that there are no conflicts of interest.










