Historical evidence suggests that tree nuts have been an important constituent of the human diet for thousands of years. It has been proposed that the use of nuts preceded cereal grains as a staple food in early civilisations( Reference Dreher, Maher and Kearney 1 ). Today, nuts continue to be essential in many cuisines around the world where they are incorporated in the diet as ingredients in recipes, as snacks, and as spreads( Reference King, Blumberg and Ingwersen 2 , Reference Jenab, Sabaté and Slimani 3 ). Nut consumption is gaining attention partly because of the increasing empirical evidence showing their beneficial effects on health and wellness( Reference King, Blumberg and Ingwersen 2 ). Findings from epidemiological and clinical studies show that nut consumption may confer beneficial effects on cardiovascular risk, type-2 diabetes and inflammation, among other health benefits( Reference Hu and Willett 4 – Reference Torabian, Haddad and Cordero-MacIntyre 9 ). These salutary effects have been linked to key nutrients present in nuts, which include vegetable protein, unsaturated fat, dietary fibre, an array of vitamins and minerals, and other bioactive compounds such as polyphenols( Reference Segura, Javierre and Lizarraga 10 ). For these reasons, various organisations including the World Health Organization( 11 ), the US Food and Drug Administration( 12 ), the UK Food Standards Agency( 13 ) and the Australian Dietary Guidelines( 14 ) recommend daily consumption of nuts in the context of a healthy diet.
In spite of the recommendation to consume nuts daily, there are still individuals that perceive nuts as fattening, hence they should be avoided or consumed sparingly( Reference Pawlak, Colby and Herring 15 ). Nuts are generally high in fat( Reference Sabaté 16 ), but consuming them in moderate amounts has been shown to have neutral or no effect on body weight( Reference Fraser, Bennett and Jaceldo 17 – Reference Sabaté, Cordero-MacIntyre and Siapco 21 ). Perhaps the focus on nuts and body weight has drawn attention away from other benefits of incorporating nuts in the diet, particularly their potential role in improving diet quality. Our group previously showed that long-term consumption of almonds induced favourable changes in nutrient composition of the diet in free-living individuals( Reference Jaceldo-Siegl, Sabaté and Rajaram 22 ). As then, other studies have observed similar findings with other types of nuts( Reference Kranz, Hill and Fleming 23 , Reference Pearson, Tey and Gray 24 ). Although these studies yielded favourable findings, they were limited by small sample sizes, inadequate dietary assessment and/or short intervention periods. Furthermore, most studies enrolled relatively younger subjects. Economic growth and technological advances of the last few decades underlie a rise in global life expectancy. This means we are living longer, healthier and more comfortable lives( Reference Goklany 25 ). However, studies show that there is a substantial decline in food intake with advancing age( Reference Wakimoto and Block 26 – Reference Morley 28 ), which is largely attributed to altered sensations of thirst, hunger and satiety affecting both the amount of food consumed and the day-to-day variations in food intake( Reference Drewnowski and Shultz 29 ). This explains why some elderly persons restrict their food choices and adopt a monotonous diet that can lead to inadequate intake of key nutrients such as protein, fibre, vitamins and non-Na minerals( Reference Drewnowski and Shultz 29 ).
We had a unique opportunity to examine dietary changes in a population of free-living older adults consuming a noticeable amount of walnuts daily as part of a 2-year trial on walnuts and age-related health outcomes. The purpose of this study was first to assess the overall nutritional quality of participants’ diet, and second, to examine nutrient displacement associated with walnut supplementation.
Methods
Study design and intervention
The Walnuts and Healthy Aging (WAHA) study from which subjects for the present study were drawn is a dual-centre (Loma Linda, California, USA and Barcelona, Spain) randomised controlled parallel trial examining the effects of daily consumption of walnuts on cognitive function and retinal health in healthy elderly subjects under free-living conditions. Our study utilised data from the Loma Linda center only. Details of the WAHA study protocol have been published( Reference Rajaram, Valls-Pedret and Cofan 30 ). In brief, cognitively healthy men and women (n 356, 65 % women) aged 63–79 years, in good general health with no uncontrolled diabetes or hypertension, were randomly assigned to either the walnut (experimental) or control group using a computerised random number with stratification by sex and age. Participants in the walnut group were asked to continue with their regular diet and in addition incorporate daily portions of packaged walnuts into their diet providing an estimated 15 % of their daily energy. Daily energy intake estimations were obtained using the WHO formula for energy for adults >60 years and the Harris Benedict Equations( Reference Movahedi 31 , Reference PinheiroVolp, Estevez de Oliveira and Duarte MoreiraAlves 32 ). Consequently, participants received 28, 42 or 56 g (1, 1·5 or 2·0 oz)/d of packaged walnuts on the basis of individual energy needs. No advice on energy restriction was given and no recipes were provided. However, participants were instructed to make-up for any day they missed ingesting the supplement by consuming twice the amount the following day. Theoretically, the experimental group represents individuals who by chance, choice or design adhere to dietary guidelines such as those set out by the American Heart Association( Reference Lichtenstein, Appel and Brands 33 ) and the World Health Organization( 11 ) by including a serving or two of nuts in their daily diet.
Participants in the control group continued with their regular or habitual diet with no supplementation and with instructions to refrain from eating walnuts and avoid compensatory intake of other nuts. No further advice on diet or lifestyle was given. In this case, the control group represents persons who by choice or otherwise do not adhere to the dietary recommendation to include nuts in their daily diet. This study was conducted according to guidelines laid down in the Declaration of Helsinki. All procedures involving human subjects were approved by the Human Subjects Committee Institutional Review Board at Loma Linda University. Written informed consent was obtained from all participants upon enrolment. WAHA study (ID NCT01634841) is registered at www.clinicaltrials.gov.
Dietary assessment
A total of 1490 unannounced telephone dietary recalls were obtained from participants during the 2-year period (752 in walnut group and 738 in control group). Most participants had five recalls each (65 %), some had four (16 %) and a few had less than four (19 %). In most cases the recalls included at least 1 weekend day. Dietary intake data were collected by trained research dietitians. Portion sizes were estimated using common household objects, for example, a deck of cards for 3 oz serving of meat, tennis ball for medium apple, compact disc to indicate diameter of one pancake. Data were analysed using Nutrition Data System for Research (NDSR) software, version 2013 developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN( 34 ). The recalls were spaced at regular intervals to capture variability and seasonality in food intake. The NDSR software utilises a multiple-pass approach to garner information about a subject’s food intake during the preceding 24 h. The method consists of three distinct passes (quick list, detailed description and review) and is designed to limit the extent of underreporting that occurs with self-reported food intake( Reference Johnson, Driscoll and Goran 35 , Reference Shim, Oh and Kim 36 ).
Telephone administered 24-h diet recalls are a valid method of assessing nutrient intake among groups of individuals and are just as effective in estimating energy intake as the recall administered in-person( Reference Tran, Johnson and Soultanakis 37 , Reference Posner, Borman and Morgan 38 ). It has the potential of markedly reducing cost, time, logistical and personnel constraints associated with dietary assessment( Reference Posner, Borman and Morgan 38 ). We have used this method successfully in previous studies( Reference Fraser, Bennett and Jaceldo 17 , Reference Wien, Oda and Sabaté 39 ).
Two measures of compliance were assessed. Unannounced 24-h telephone dietary recalls and a biomarker. The dietary recalls were used to determine if subjects in the walnut group consumed their allotted amount of walnuts, and if their counterparts in the control group refrained from deliberate consumption of walnuts. For the walnut group, consumption of walnuts 6–7 d/week (85–100 %) was considered excellent compliance and 4–5 d/week (57–71 %) good compliance. Those who consumed walnuts ≤3 d were determined to be non-compliant. In the control group, participants were deemed fully compliant if they refrained from eating walnuts in any of the recalls, or if they consumed no more than 15 g walnuts on any given day.
Statistical analysis
The arithmetic mean daily intake of energy and key nutrients from valid 24-h telephone diet recalls was calculated for each participant. Those with less than two recalls were excluded from analysis. The distributions for energy, macronutrients and select micronutrients were not normal, so we used log transformation to better approximate normality except for total PUFA and cholesterol, for which we used square root transformation. All values were back transformed. The independent samples t test was used to test the difference in energy and nutrient intake between the walnut and the control groups. Statistical significance was defined by a P≤0·05. Results are presented as mean values and standard deviations or as mean values with their standard errors. All analyses were performed using Statistical Packages for Social Sciences (SPSS version 23; IBM Corp.).
A sample size of 160 with randomisation to two equal groups was estimated to provide sufficient power to detect a 5 % difference in energy and protein intake between the two groups. This corresponds to 460 (sd 552) kJ (110 (sd 132) kcal) for energy and 5 (sd 7) g for protein. The final sample size of 317 showed that we had power in excess of 95 %.
Estimation of nutrient displacement
We followed the method previously used by our group to calculate energy and nutrient displacement( Reference Jaceldo-Siegl, Sabaté and Rajaram 22 , Reference Alshaeri, Natto and Tonstad 40 ). Displacement is defined as ‘the inverse measure of the degree to which a food supplement induces change in the content of a particular nutrient in the supplemented diet’( Reference Jaceldo-Siegl, Sabaté and Rajaram 22 ). The concept of displacement was first used in a study where dietary intake information was collected before and after the intervention, hence each subject acted as his/her own comparator. We propose that the displacement concept is even more relevant in a parallel design study with a concurrent control group. In our study, the control diet (CD) not only represents the habitual diet of participants, but is also the best estimate of the diet of participants in the walnut group before the intervention. The actual walnut diet (aWD) represents a modified habitual diet that now includes walnuts. As the walnut supplement (W) was added to the habitual diet, we can compute the expected intake of a nutrient by taking the control diet and adding a nutrient present in the walnut supplement. This is referred to as the expected walnut diet (eWD), and is in essence what we would expect if there was no displacement. Displacement (D) of a nutrient therefore is the difference between the expected supplemented (walnut) diet and the actual supplemented (walnut) diet. This can be expressed as a percentage by dividing the displaced nutrient by the amount of the same nutrient present in the walnut supplement multiplied by 100:
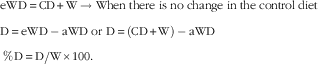 $$\eqalignno{ & {\rm eWD}\, {\equals}\, {\rm CD}{\plus}{\rm W}\to{\rm When}\;{\rm there}\;{\rm is}\;{\rm no}\;{\rm change}\;{\rm in}\;{\rm the}\;{\rm control}\;{\rm diet} \cr & {\rm D}\, {\equals}\, {\rm eWD}-{\rm aWD}\;{\rm or}\;{\rm D}\, {\equals}\, \left( {{\rm CD}{\plus}{\rm W}} \right)-{\rm aWD} \cr & {{\,\%\,{\rm D}\, {\equals}\, {\rm D}} \mathord{\left/ {\vphantom {{\,\%\,{\rm D}\, {\equals}\, {\rm D}} {{\rm W}{\times}100}}} \right. \kern-\nulldelimiterspace} {{\rm W}{\times}100}}. $$
$$\eqalignno{ & {\rm eWD}\, {\equals}\, {\rm CD}{\plus}{\rm W}\to{\rm When}\;{\rm there}\;{\rm is}\;{\rm no}\;{\rm change}\;{\rm in}\;{\rm the}\;{\rm control}\;{\rm diet} \cr & {\rm D}\, {\equals}\, {\rm eWD}-{\rm aWD}\;{\rm or}\;{\rm D}\, {\equals}\, \left( {{\rm CD}{\plus}{\rm W}} \right)-{\rm aWD} \cr & {{\,\%\,{\rm D}\, {\equals}\, {\rm D}} \mathord{\left/ {\vphantom {{\,\%\,{\rm D}\, {\equals}\, {\rm D}} {{\rm W}{\times}100}}} \right. \kern-\nulldelimiterspace} {{\rm W}{\times}100}}. $$
Fig. 1 illustrates the concept of ‘displacement’. A displacement estimate of 100 % means that a nutrient in walnuts reduced by an equal amount the intake of the same nutrient from other food sources in the actual walnut diet, thus the net effect of supplementation is zero change in total intake of that nutrient. A value between 0 and 100 % indicates partial displacement, which means that intake of the nutrient in question from non-walnut food sources is reduced, however, the net effect of supplementation is an overall increase in intake of that nutrient in the actual walnut diet. Displacement of more than 100 % means that non-walnut foods provided less of that nutrient and now the overall supplemented diet has less of this nutrient. A negative percentage displacement results when the non-walnut foods in the walnut diet provide more of the nutrient than in the habitual or control diet.

Fig. 1 Illustration of the concept of ‘displacement’ with a hypothetical example of energy displacement by a 1151 kJ walnut supplement. CD, habitual diet; W, walnut supplement; aWD, walnut diet.
Results
Participants
Table 1 shows the baseline characteristics of 317 participants (63·4 % female; 50·8 % walnut group) who had sufficient data for analysis. The average age of the participants was 69·6 years in the walnut group and 69·1 years in the control group. Both treatment groups were comparable regarding height, body weight, body composition parameters, sex and ethnicity. We excluded from analysis thirty-nine participants who dropped out of the study in the first few months and for whom dietary data were unavailable. There were twenty dropouts in the walnut group and nineteen in the control. The dropouts did not differ significantly from completers on age, sex, ethnicity and baseline BMI.
Table 1 Baseline characteristics of the Walnuts and Healthy Aging study participants according to treatment allocation (Mean values and standard deviations; numbers and percentages)
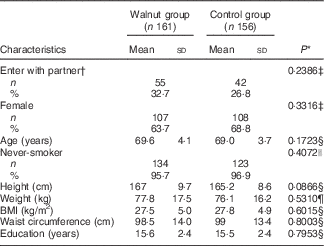
* P value for comparison between the walnut and control groups.
† Subjects who entered the study with a spouse, partner or friend and were randomised into the same group.
‡ χ 2 test for independence.
§ Two-sample t test.
|| Fisher’s exact test.
¶ Mann–Whitney test.
Compliance on the intervention
Out of 738 dietary recalls obtained from subjects in the control group, none showed conscious consumption of walnuts. Intake of trivial amounts of walnuts (<15 g) was reported in only seven (<1 %) dietary recalls, mostly as an ingredient in recipes and commercially prepared foods such as walnut bread, cookies and salads. We therefore consider subjects in the control group to have been fully (100 %) compliant with instructions not to consume walnuts. Similarly, of the 752 dietary recalls obtained from subjects in the walnut group, 744 (99 %) reported consumption of the prescribed amount of walnuts (between 28 and 56 g). The average daily intake of walnuts as reported in the 24-h recalls was 43 g for the walnut supplemented diet and only 0·7 g for the control diet. The self-reported intake or no intake of walnuts was reflected by changes in erythrocyte α-linolenic acid (ALA), a biomarker of walnut intake and which we have used in our previous studies( Reference Sabaté, Cordero-MacIntyre and Siapco 21 , Reference Sabate, Fraser and Burke 41 ). Data from a subsample of study participants showed that both groups started the study with nearly similar mean proportions (of total erythrocyte fatty acids) of ALA, 0·31 mmol/l for the walnut group and 0·29 mmol/l for the control group (P=0·167). However, a year into the trial, the mean ALA increased by 28 % in the walnut group, whereas it decreased by 17 % in the control group (P<0·001)( Reference Huey, Bitok and Kazzi 42 ). At the end of the study, the average walnut intake and erythrocyte ALA were significantly correlated (r 0·61, P<0·001), as was walnut intake and change in erythrocyte ALA from baseline (r 0·49, P<0·001). The decrease in erythrocyte ALA in the control group may have resulted from advice to participants to restrict the use of flax products, especially flax supplements which are known to contain significant amounts of ALA.
Nutrient profile
The first two columns of Table 2 show crude values for energy and selected nutrients in the walnut and control groups. The prescribed walnut supplement provided approximately 1176 kJ (281 kcal) or 14·5 % of estimated mean daily energy. Overall, the walnut group had greater intake of all reported macro- and micronutrients except for Na and Se. Fig. 2 and 3 show the same data standardised for differences in energy intake. Following standardisation, the walnut group had significantly higher intake of total fat, vegetable protein, total PUFA, n-3 PUFA, n-6 PUFA, Mg, Cu and Mn, whereas the control group consumed significantly more carbohydrate, SFA, Na, animal protein, thiamin, riboflavin and vitamin E. There were no significant differences between the two groups in intake of Ca, Zn, K, folate, and vitamins B6, B12 and D.

Fig. 2 Mean daily intake of dietary macronutrients after a 2-year supplementation with 43 g of walnuts. ![]() , Walnut diet;
, Walnut diet; ![]() , control diet. † Values represent mean intake/4184 kJ of five 24-h diet recalls obtained over the 2-year study period. * P<0·05; ** P<0·001 (t test).
, control diet. † Values represent mean intake/4184 kJ of five 24-h diet recalls obtained over the 2-year study period. * P<0·05; ** P<0·001 (t test).

Fig. 3 Mean daily intake of dietary micronutrients after a 2-year supplementation with 43 g of walnuts. ![]() , Walnut diet;
, Walnut diet; ![]() , control diet. † Values represent mean intake/4184 kJ of five 24-h diet recalls obtained over the 2-year study period. * P<0·05; ** P<0·001 (t test).
, control diet. † Values represent mean intake/4184 kJ of five 24-h diet recalls obtained over the 2-year study period. * P<0·05; ** P<0·001 (t test).
Table 2 Displacement of select nutrients after a 2-year supplementation with 43 g walnuts

aWD, intake of a nutrient in the walnut supplemented diet; CD, intake of a nutrient in the control diet; W, mean intake of walnuts in the walnut supplemented diet; eWD, expected walnut diet; D, displacement of that nutrient.
* Mean values (g) for five 24-h diet recalls per individual.
† Percentage differences.
Energy and nutrient displacement
Table 2 presents the displacement of energy and nutrients. The mean total energy intake in the actual walnut diet was 222 kJ (53 kcal) lower than in the expected walnut diet with a computed displacement of 18·9 %. This means that 19 % of the energy provided by other energy-containing foods in the diet was displaced by walnuts. Similar displacements were observed for total protein (24·2 %), total fat (25 %), MUFA (21 %), total PUFA (16 %), n-6 PUFA (17 %), n-3 PUFA (12·8 %) and insoluble fibre (9 %). The displacement of SFA was higher compared with other fatty acids (62 %). There was a negative displacement for total carbohydrate, total fibre, and soluble fibre, indicating that non-walnut foods in the walnut supplemented diet provided more of these nutrients than in the control group even after being supplemented by the walnuts. This was also true for vitamins A, K, C and Ca, that is, a net increase from non-walnut food sources compared with control group. Displacement estimates for niacin, Na and Se were all >100 %, indicating that the walnut supplement more than fully displaced these nutrients from the diet and as a consequence the actual walnut diet contained less of these nutrients.
The walnut diet met the Acceptable Macronutrient Distribution Ranges (AMDR) for total digestible carbohydrates, total protein and amino acids, MUFA, total fibre and added sugars, but exceeded the recommendations for total fat (6·5 %), SFA (0·9 %), PUFA (5·3 %) and Na (67 %). The control diet met the AMDR for total digestible carbohydrates, total protein and amino acids, MUFA, PUFA, total fibre and added sugars, but exceeded the recommendations for total fat (0·6 %), SFA (1·9 %) and Na (67 %) (Fig. 4).

Fig. 4 Nutrient intake in the walnut (![]() ) and control diets (
) and control diets (![]() ) as a percentage of goal or limit. % E, percentage of total energy intake. * Dietary Reference Intakes: Acceptable Macronutrient Distribution Ranges (Food and Nutrition Board, Institute of Medicine, National Academies). † Dietary Reference Intakes: Recommended Dietary Allowances and Adequate Intakes, Elements (Food and Nutrition Board, Institute of Medicine, National Academies).
) as a percentage of goal or limit. % E, percentage of total energy intake. * Dietary Reference Intakes: Acceptable Macronutrient Distribution Ranges (Food and Nutrition Board, Institute of Medicine, National Academies). † Dietary Reference Intakes: Recommended Dietary Allowances and Adequate Intakes, Elements (Food and Nutrition Board, Institute of Medicine, National Academies).
Discussion
The results of this study demonstrate that prescribing a daily supplement of walnuts in the diet of elderly free-living subjects on self-selected diets can lead to an improvement in nutrient intake in a way that promotes cardiovascular health. Compared with controls, subjects consuming walnuts daily reported significantly higher intake of total protein, vegetable protein, total fat, total PUFA, n-3 PUFA, n-6 PUFA, Mg, Cu, and Mn and significantly lower intakes of total carbohydrate, animal protein, SFA and Na. Limiting animal protein and increasing vegetable protein in the diet has been shown to help in reducing SFA intake and is often a recommended strategy for reducing cholesterol intake( Reference Shikany and White 43 ). We consider that the lower intake of SFA in the walnut diet is as a result of replacing foods high in SFA, such as meats and full-fat dairy products, with high protein vegetables, legumes and nuts (walnuts). The increase in total fat intake in the walnut diet can be attributed to higher intake of total PUFA, both n-6 and n-3. Findings from a systematic review and meta-analysis of randomised controlled trials focusing on dietary PUFA and clinical CHD events show that consuming PUFA in place of SFA reduces risk( Reference Mozaffarian, Micha and Wallace 44 ). The same study also found no evidence for increased risk in long-term trials utilising PUFA consumption at levels as high as a mean 14·9 % energy, suggesting that the current recommendation for an upper limit of PUFA consumption at 10 % may need to be revised upwards( Reference Mozaffarian, Micha and Wallace 44 ). The average Na intake in both the walnut and control diets was well above the recommendation for older adults. Nevertheless, intake in the walnut diet was significantly lower when compared with control.
When compared with age-specific US Dietary Reference Intakes, PUFA and total fat (as % of energy) intake was significantly higher in the walnut diet than in the control. This is largely because of the PUFA contribution from the walnut supplement. The percentage of total energy derived from carbohydrate and SFA was significantly higher in the control than in the walnut diet. There were no significant differences between the two diets in percentage of total energy derived from protein and MUFA. The walnut supplemented diet met the AMDR values for total protein and amino acids, SFA, MUFA, cholesterol, fibre and added sugars but exceeded values for total fat, PUFA and Na. It must be noted, however, that intake of Na in both study groups exceeded the recommended levels, mirroring the general trend in Na intake in the US population( Reference Zhang, Cogswell and Gillespie 45 ).
The concept of energy and nutrient ‘displacement’ as a result of nut consumption was first demonstrated by Fraser et al. ( Reference Fraser, Bennett and Jaceldo 17 ) and Jaceldo-Siegl et al.( Reference Jaceldo-Siegl, Sabaté and Rajaram 22 ) and has been applied to other foods other than nuts( Reference Wien, Oda and Sabaté 39 ). Jaceldo-Siegl et al. reported that free-living subjects consuming 54 g of almonds a day for 6 months displaced more than one-half of the total energy, one-third of the protein, one-quarter of total fat, 98 % SFA, 16 % MUFA and 26 % PUFA from their habitual diet. In addition, total fibre, Ca, iron, Mg, P, K and Zn were partially displaced. A similar study conducted by Kranz et al. ( Reference Kranz, Hill and Fleming 23 ) showed that supplementing the usual diet of men with 75 g of walnuts for 8 weeks under free-living conditions resulted in displacement of energy (25 %), total fibre (61 %), total fat (15 %) and SFA (15 %) from non-walnut food sources. More recently, Pearson et al.( Reference Pearson, Tey and Gray 24 ) noted that following 12-week consumption of hazelnuts, 68 % energy compensation occurred though this compensation level did not differ significantly with other energy-dense snacks. Other nutrients displaced by the hazelnuts were protein (30 %), total fat (10 %), MUFA (22 %), PUFA (8 %) and SFA (165 %). Collectively, the results of these studies and ours indicate that supplementation with various tree nuts consistently resulted in partial displacement of energy, protein, total fat, MUFA and PUFA. However, displacement estimates for other nutrients such as SFA, carbohydrate, fibre, vitamin C, Ca, K, and Mg tended to differ amongst the studies. In general displacement patterns tend towards a healthier nutrient profile with the nut supplement.
The relationship between energy compensation and changes in body weight is of significant interest in nut studies. Although there was a narrow difference in energy compensation between our study and that of Kranz et al. (19 and 25 %, respectively), our participants had a tendency to lose weight (−0·4 kg for walnut group and −0·7 kg for control, P value for group difference>0·05), unlike those of Kranz et al. who maintained theirs. Needless to say, unintentional weight loss is a common phenomenon among older adults( Reference Stajkovic, Aitken and Holroyd-Leduc 46 ). The studies by Jaceldo-Siegl et al. and Pearson et al. report higher energy compensation estimates (54 and 68 %, respectively) with non-significant increase in body weight (+0·4 and +0·8 kg, respectively). There are two possible reasons that may explain the relatively lower energy compensation observed in our study. First and foremost, unlike younger individuals, older adults (for social, monetary or health-related reasons) tend to have well established and stable eating patterns( Reference Kumanyika, Tell and Shemanski 47 ). Thus it is very likely that the majority of our study participants maintained their habitual eating habits and simply added walnuts to their diet. Second, individual study results may have been influenced by the study design used. Most of the studies that examined food/nutrient replacement with nut intake used the pre-post or within subject design (sequential or crossover), unlike our study that used the parallel design.
Displacement estimates for total protein, total fat, SFA, MUFA, thiamin, B6, Mg, Fe, Zn, and Cu showed partial compensation, suggesting that there was an overall increase in intake of these nutrients as a result of both the walnut supplement and contribution from other food sources in the diet. Although intake of total PUFA, n-3 and n-6 PUFA was significantly higher in the walnut diet, displacement estimates are marginal, suggesting that participants substituted walnuts for other PUFA-rich foods, although to a lesser extent. Se and Na showed overcompensation with values of 309 and 1600 %, respectively. These values are much higher than previously reported( Reference Jaceldo-Siegl, Sabaté and Rajaram 22 ). Displacement estimates for carbohydrate, total and soluble fibre, vitamin K, Ca and P were all negative, suggesting that not only was there no displacement, but that non-walnut foods in the walnut supplemented diet contained more of these nutrients than in the control diet. This is not unexpected, particularly as walnuts contain very little of these nutrients. More importantly, however, is that this could be an indication of how the walnut supplement was consumed. It is conceivable that the supplement may have been consumed together with other foods such as breakfast cereal, fruit smoothies, salads or dairy products such as ice-cream and yogurt, which are good sources of these nutrients.
Strengths and limitations
To the best of our knowledge, this study has the largest sample size and longer duration than any previously published study that has examined nutrient displacement ensuing consumption of nuts or any other food. The approach we used in the current study assesses nutrient displacement at the group level. This design is advantageous as it has the ability to capture seasonal oscillations in food intake while keeping secular trends and influences to be the same in both groups. We are aware that the use of self-reported dietary assessment methods is subject to built-in errors of over- and underreporting. In our study, both groups were assessed with the same method, hence we believe that inherent measurement errors were uniformly distributed in the two groups. We also acknowledge that nutrient status of an individual is determined by many factors, including absorption efficiency, and the self-reported intake of nutrients may not necessarily translate to changes in nutrient status. The original study was designed to assess changes in cognitive function and retinal health and our results derives from a post hoc analysis.
Conclusion
The results of this study adds to the body of evidence linking nuts and chronic disease by showing that subjects on self-selected diets with inclusion of walnuts had favourable nutrient profiles compared with those on self-selected diets without walnuts. This affirms that which has already been shown in epidemiological studies that including nuts as part of the daily diet contributes to chronic disease prevention. We are aware that the concept of displacement may not apply to everyone and some populations may require specific guidelines and strategies for incorporating walnuts into the diet. We hope that findings from this study will help clinicians and nutrition educators make appropriate recommendations for incorporating walnuts into the diet of older adults. We conclude therefore that a daily supplement of walnuts can induce favourable modifications to the nutrient profile of older adults in a manner that promotes overall health and cardiovascular health in particular.
Acknowledgements
The authors thank Natalie Kazzi and Lynnley Huey for their assistance in collection of dietary data, and Rawiwan Sirirat for her assistance with quality control on 24-h telephone diet recalls. Ciber Fisiopatología de la Obesidad y Nutrición is an initiative of Instituto de Salud Carlos III, Spain.
This work was supported by a grant from the California Walnut Commission, Sacramento, CA, USA. The funding agency had no role in the study design, data collection, analyses, interpretation of data or in writing this article.
The authors’ responsibilities were as follows: J. S. obtained the funding; K. J.-S., J. S., E. B. designed the study; E. B. collected and analysed the data and drafted the paper; E. R., S. R., M. S.-M., I. R. and T. F.-S. provided input on the paper. J. S. and K. J.-S. edited the final version. All the authors read and approved the final version of the manuscript.
The Principal Investigators of the study (J. S. and E. R.) have received grants for research through their institutions from the California Walnut Commission and are non-paid members of its Scientific Advisory Committee. The authors declare that there are no conflicts of interest.










