The management of individuals with moderate hypercholesterolaemia in primary prevention constantly poses the question whether it is more acceptable to treat with drugs or with an appropriate diet(Reference Houston, Fazio and Chilton1, Reference Sirtori, Galli and Anderson2). In fact, although low-risk individuals with accompanying features, such as elevated inflammatory markers, could possibly benefit from statins, as shown, for example, in the JUPITER (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) trial(Reference Fonseca and Izar3), the evidence is less clear for individuals with a baseline Framingham risk score between 5 and 10 % over 10 years and with LDL-cholesterol (LDL-C) levels below 1600 mg/l (ASCOT-LLA(Reference Sever, Dahlöf and Poulter4), ASPEN(Reference Knopp, d'Emden and Smilde5) and FIELD (Fenofibrate Intervention and Event Lowering in Diabetes)(Reference Keech, Simes and Barter6) studies). In these cases, concern might be raised by the potential muscular side effects of statins(Reference Sinzinger and O'Grady7) as well as by the costs(Reference Baigent, Keech and Kearney8).
A well-planned diet may be quite effective: the most extreme example is provided by the ‘portfolio diet’ approach developed by Jenkins et al. (Reference Jenkins, Kendall and Faulkner9). In severe hypercholesterolaemic individuals, this diet, containing vegetable proteins, viscous fibres, phytosterols and tree nuts, was as effective as a mid-dose statin(Reference Jenkins, Kendall and Marchie10). However, the long-term compliance of such a diet may be problematic.
Thus, besides a ‘healthy diet’ regimen, generally resulting in LDL-C reductions of < 5 %(Reference Kris-Etherton, Etherton and Carlson11), it may be useful to advise patients on functional foods or dietary supplements(Reference Sirtori, Anderson and Arnoldi12). In this field, soya protein has attracted considerable interest, since the early demonstration in 1977 of its cholesterol-reducing properties(Reference Sirtori, Agradi and Conti13). This initial report was followed by numerous investigations summarised in a meta-analysis(Reference Anderson, Johnstone and Cook-Newell14), indicating a particular sensitivity of hypercholesterolaemic individuals to this protein. However, in the following years, these results were confirmed by other groups(Reference Jenkins, Mirrahimi and Srichaikul15), with the limitation that patients with severe hypercholesterolaemia could not be involved, as the treatment of these subjects with drugs has become compulsory(Reference Sirtori, Eberini and Arnoldi16). A recent review(Reference Harland and Haffner17) has confirmed that consuming 25 g of soya protein instead of animal proteins in the diet of adults with normal or mild hypercholesterolaemia results in significant reductions in total and LDL-C, equivalent to 6 % LDL-C reduction.
Some recent papers suggest that the proteins of other grain legumes may possess similar activities(Reference Bazzano, Thompson and Tees18, Reference Sirtori, Galli and Anderson19). For example, investigations on a rat model of hyperlipidaemia have shown that pea proteins(Reference Rigamonti, Parolini and Marchesi20, Reference Spielmann, Stangl and Eder21) and lupin proteins(Reference Sirtori, Lovati and Manzoni22, Reference Bettzieche, Brandsch and Weisse23) are effective in reducing total cholesterol and LDL-C. In addition, a recent study on a rabbit model of atherosclerosis has shown that the latter is also able to slow the formation of atherosclerotic plaques(Reference Marchesi, Parolini and Diani24). Clinical studies on these innovative plant proteins are, instead, still scarce(Reference Hodgson, Lee and Puddey25–Reference Nowicka, Klosiewicz-Latoszek and Sirtori28).
The main objectives of the present work were twofold: (1) to investigate the potential cholesterol-lowering activity of lupin or pea proteins in hypercholesterolaemic subjects; (2) to verify whether combinations of one plant protein and one soluble fibre in the same food product may provide an improved cholesterol-lowering activity. The selected soluble fibres were oat fibre(Reference Anderson, Spencer and Hamilton29, Reference Queenan, Stewart and Smith30) and apple pectin(Reference González, Rivas and Caride31), two fibres with different structural characteristics. Owing to fund limitation, it was possible to test combinations including only one plant protein.
A double-blind clinical trial was thus planned, with a parallel design: the participants were treated with dietary bars containing the protein/fibre combinations for 4 weeks, casein being the control protein and cellulose the control fibre. The present study was carried out in a single centre with a large input of hypercholesterolaemic participants.
Subjects, materials and methods
Participants
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki. Participants were recruited among those attending the University Centre for Dyslipidaemias of the Niguarda Hospital (Milano, Italy), according to the following criteria: (1) males and postmenopausal females; (2) participants in primary prevention, non-diabetics; (3) total cholesterol >2200 mg/l and TAG < 2000 mg/l. Only participants free from any lipid-lowering drug and not affected by any kind of food allergy were enrolled. All procedures were in accordance with the ethical standards of the institution on human experimentation and, before starting, the study was approved by the Ethics Committee of the Niguarda Hospital. The participants were carefully informed about the modalities and end points of the present study and, after receiving all necessary information, they signed their written informed consent.
Design
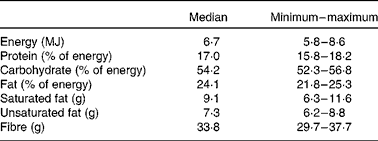
A randomised, double-blind, parallel group design was followed, with total cholesterol as the main end point. All the candidates underwent a stabilisation period on a hypolipidaemic dietary regimen for 4 weeks (Table 1). Dietary intakes were assessed using a 3 d food diary designed by a qualified dietitian. After this period, a baseline blood sample was drawn. All participants showing cholesterol changes exceeding ± 10 % were excluded from the present study. The selected subjects were randomised into seven dietary treatment groups: each group consumed two bars per day containing the specific protein/fibre combination for 4 weeks. The first group consumed casein as the protein and cellulose as the fibre (control); in the second and the third groups lupin or pea protein replaced casein and the fibre was cellulose; the fourth and fifth groups consumed casein as the protein and oat fibre and apple pectin as the fibre, respectively; the sixth and seventh group received combinations of pea protein with oat fibre and apple pectin, respectively. At the starting visit (time 0), all randomised subjects received their complete bar supplies for the treatment period. The composition of the total diet during the present study is reported in Table 1. At the end of this, participants were examined by an attending physician, recording body weight and measuring blood pressure with the subject in sitting position. A final blood sample was recovered for the evaluation of the lipid, metabolic and inflammatory changes after treatment.
Table 1 Nutrient intake during the study
(Medians, and minimum and maximum values)
Composition of the dietary bars
The dietary bars, whose composition is given in Table 2, were prepared by the Fraunhofer Institute (IVV, Freising, Germany). The main ingredients were a protein (casein, pea protein or lupin protein) and a fibre (cellulose, oat fibre or apple pectin), whereas the other ingredients listed in the table were added to obtain suitable texture and taste; the bars with casein contained a larger amount of water in order to allow an easier chewing. Sodium sorbate was added as a preserving agent and, immediately after production, the packed bars were sealed under a modified atmosphere.
Table 2 Total components of the two bars that each participant consumed per day
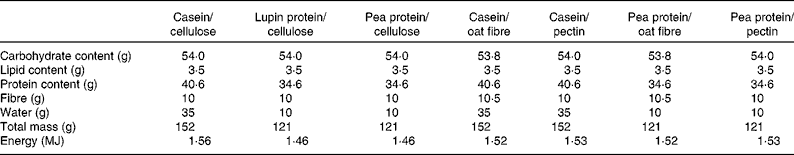
Pea protein was a commercial protein isolate from seeds of Pisum sativus (Pisane C9; Provital Industrie SA, Pecq, Belgium), constituted by 90·8 % pea protein on DM and low amounts of fat (1·2 %) and fibre (0·9 %). The lupin protein isolate was prepared by the Fraunhofer Institute starting from deoiled Lupinus angustifolius cultivar Boregine, consisting of 91·7 % lupin protein on DM and low amounts of fat (1·16 %) and ash (3·39 %). The production procedure is described elsewhere(Reference D'Agostina, Antonioni and Resta32). The oat fibre was an experimental ingredient containing 25–28 % β-glucan provided by CreaNutrition AG (Zurich, Switzerland), whereas apple pectin was a high methylester pectin from apple peels (degree of esterification 68–78 %, Pectine Classic AU201 ESP; Herbstreith & Fox, Neuenbürg, Germany).
Laboratory procedures
Blood samples were collected after an overnight fast. Both serum and EDTA plasma were prepared by low-speed centrifugation at 4°C and stored at − 80°C. Plasma total cholesterol, TAG, HDL-cholesterol and glucose concentrations were determined with standard enzymatic techniques on a Roche Diagnostics Cobas 400 Analyser (Roche Diagnostics GmbH, Mannheim, Germany). Plasma LDL-C was calculated with Friedewald's formula. High-sensitivity C-reactive protein concentrations were measured by immunoturbidimetry on a Roche Diagnostics Cobas 400 Analyser (Roche Diagnostics GmbH). Plasma levels of the soluble forms of intracellular cell adhesion molecule-1, IL-6 and adiponectin were measured by using commercially available monoclonal antibody-based ELISA kits (R&D Systems, Minneapolis, MN, USA). Serum insulin levels were determined by a solid-phase enzyme-amplified sensitivity immunoassay performed on breakable microtiter plates (Biosource International, Camarillo, CA, USA). The assays were performed in duplicate for each sample. The homeostasis model assessment of insulin resistance index was calculated as proposed by Matthews et al. (Reference Matthews, Hosker and Rudenski33).
Statistical analysis
Results were expressed as mean values and standard deviations, if not otherwise stated. Data with skewed distributions (TAG) were log-transformed before analyses. Changes caused by each treatment were analysed by using the paired t test. Multiple comparison analysis was performed by ANOVA with Dunnett's test, followed by adjustment for baseline values, for comparisons of the six treated groups v. the control group (casein/cellulose). Group differences with P < 0·05 were considered as statistically significant.
Results
The participant flow diagram is shown in Fig. 1. In total, 271 subjects were evaluated in order to find the most appropriate candidates. At the end, 193 subjects were selected and evaluated before and after a run-in period on the standard dietary regimen for 4 weeks. After the stabilisation period, two were non-compliant and sixteen subjects had cholesterol changes exceeding ± 10 % and were therefore excluded. A total of 175 participants were thus randomised into seven dietary treatment groups. The demographic and anthropometric characteristics and the lipid values measured at randomisation in participants participating in the present study are shown in Table 3. The comparison of baseline values by ANOVA revealed a borderline significant difference among groups. In total, seventeen subjects dropped out from the present study: three because of health problems (one because of vomiting, one because of nausea and one because of minor gastrointestinal side effects) and fourteen because they considered the bars unsatisfactory when consumed daily. The vast majority were, however, satisfied with the product they had received. There were no significant body weight changes during the treatments and, in particular, no subject reported any relevant body weight reduction (data not shown).

Fig. 1 Participation flow throughout the trial. prot., protein.
Table 3 Baseline characteristics of the participants
(Mean values, standard deviations and number of participants)

SBP, systolic blood pressure; DBP, diastolic blood pressure.
The plasma total cholesterol changes are reported in Table 4. There were no cholesterol changes in the control diet (casein+cellulose), a fact that strongly supports the reliability of the present study. Statistically significant total cholesterol reductions were observed in the case of casein+apple pectin ( − 152 mg/l, − 5·3 %), lupin protein+cellulose ( − 116 mg/l, − 4·2 %), pea protein+oat fibre ( − 135 mg/l, − 4·7 %) and pea protein+apple pectin ( − 168 mg/l, − 6·4 %). When all the groups were compared by ANOVA with the Dunnett's test, followed by adjustment for baseline values, the association of pea protein+apple pectin showed the most statistically significant reduction (P = 0·0098 v. control).
Table 4 Effects of treatments on lipid parameters
(Mean values and standard deviations)

* Mean values were significantly different from those of baseline (P < 0·05).
Data on LDL-C were mainly confirmatory: the combinations lupin protein+cellulose and casein+apple pectin were slightly active, but the changes were not statistically significant, whereas the combinations of pea protein+oat fibre and pea protein+apple pectin determined a statistically significant decrease ( − 118 mg/l, − 5·8 % and − 106 mg/l, − 9·2 %, respectively). When all the groups were compared by ANOVA with the Dunnett's test, followed by adjustment for baseline values, the association of pea protein+apple pectin showed the most statistically significant reduction (P = 0·004 v. control).
HDL-cholesterol changes (Table 4) were negligible; in general, there were small reductions (mean − 12 mg/l) randomly distributed among the different treatment groups. Similarly, TAG changes were again minimal, some treatments being followed by modest increases (e.g. pea protein+apple pectin) or reductions (e.g. lupin protein+cellulose).
The homeostasis model assessment of insulin resistance index values were significantly reduced after consumption of casein+cellulose, casein+pectin and pea protein+oat fibre. This was mainly dependent on the insulin level reductions, whereas only the last combination was able to decrease glucose concentrations also (Table 5). On the whole, the most promising combination was pea protein+oat fibre, as the participants showed 4 % glucose reduction, 57 % insulin reduction and 25 % decrease of the homeostasis model assessment of insulin resistance index. Adiponectin and the inflammatory markers remained essentially constant with all the treatments (Table 5).
Table 5 Effects of bioactive fibres and proteins on metabolic and inflammatory parameters
(Mean values and standard deviations)

HOMA-IR, homeostasis model assessment of insulin resistance; sICAM-1, soluble intracellular cell adhesion molecule-1; Hs-CRP, high-sensitivity C-reactive protein.
* Mean values were significantly different from those of baseline (P < 0·05).
† 1μU/ml = 6·945 pmol/l.
Discussion
In the present clinical trial, dietary bars containing different mixtures of plant proteins and soluble fibres were tested with the objective of developing new strategies for the prevention and the long-term control of mild hypercholesterolaemia based on innovative ingredients and combinations. The present study was carried out in a single clinical centre and included participants with a low cardiovascular risk and those willing to undergo a non-drug treatment. The present study was conducted in favourable conditions, as most participants were well known by the personnel of the centre; the diet of each individual was planned in detail by an expert dietitian and, finally, all participants who, after the run-in period, showed cholesterol changes exceeding ± 10 % were excluded. In addition, although the lower limit of cholesterolaemia for patient selection was 2200 mg/l, the actual baseline averages of all groups were >2600 mg/l. The reliability of the present study is confirmed by the fact that the lipid parameters of the reference group at the end of the study were essentially equal to the baseline values.
The objectives of the work were twofold: the former was to determine the cholesterol-lowering activity of lupin and pea protein, never investigated before in hypercholesterolaemic human subjects; the latter was to assess the potential efficacy of combinations of a plant protein and a soluble fibre.
The efficacy of each plant protein may be evaluated from the results of the group consuming plant proteins+cellulose combinations. There is a difference between pea protein, which was inactive, and lupin protein, which appeared to be moderately active. This difference was unexpected on the basis of the outcomes of studies based on the rat model of hyperlipidaemia, which gave equivalent results for pea protein isolates(Reference Rigamonti, Parolini and Marchesi20, Reference Spielmann, Stangl and Eder21) and lupin protein isolates(Reference Sirtori, Lovati and Manzoni22, Reference Bettzieche, Brandsch and Weisse23). The observed decrease in total cholesterol levels ( − 116 mg/l, − 4·2 % change) in the group consuming the lupin protein/cellulose bar is in the same range of the cholesterol change observed when treating participants with comparable baseline cholesterolaemia with 25 g/d of soya protein(Reference Jenkins, Mirrahimi and Srichaikul15, Reference Harland and Haffner17). On the contrary, in a previous study, where normocholesterolaemic subjects consumed a lupin-enriched diet for 12 months, lupin did not appear to decrease cholesterol(Reference Belski, Mori and Puddey27). As in the case of soya, legume proteins are effective mainly in hypercholesterolaemics(Reference Anderson, Johnstone and Cook-Newell14).
As a first step to evaluate the efficacy of plant proteins and soluble fibres, it was also necessary to test casein+soluble fibre combinations. In our conditions, only pectin produced a statistically significant decrease of total cholesterol ( − 5·3 %), accompanied by a non-statistically significant decrease of LDL-C, whereas oat fibre resulted to be inactive. This outcome may be possibly explained by the moderate amount of β-glucan consumed by the subjects (only 2·8 g/d), as, in general, at least 3 g of this ingredient is needed to observe a clear effect(Reference Brown, Rosner and Willett34). There is still debate on the actual efficacy of soluble fibres in cholesterol reduction, and the reported variability is in any case very large: the range of effects on total cholesterol varies, in fact, from 0 to − 18 % for oat products, from − 5 to − 16 % for pectin, from 3 to − 17 % for psyllium and from 4 to − 17 % for guar gum(Reference Kris-Etherton, Krummel and Russell35). Several reasons may explain such variability and certainly some fibres are more effective than others; for example, psyllium-enriched cereals lower cholesterol more effectively than pectin-enriched ones(Reference Bell, Hectorn and Reynolds36). In addition, the response depends on the baseline cholesterol value, hypercholesterolaemic individuals being much more sensitive than normolipidaemic individuals(Reference Anderson, Spencer and Hamilton29, Reference Ripsin, Keenan and Jacobs37).
The strategy of combining a plant protein and a soluble fibre appeared to be successful, as the cholesterol reductions observed with pea protein+oat fibre (total cholesterol change − 4·7 %, LDL-C change − 5·8 %) or pea protein+apple pectin (total cholesterol change − 6·4 %, LDL-C change − 9·2 %) were larger than those of each single ingredient. The potential health benefits of the latter combination also were confirmed by the observed decreases of glucose, insulin and homeostasis model assessment index. The observed LDL-C change falls in the high range of recent trials on soya protein(Reference Sirtori, Galli and Anderson38) and is comparable to the activity of phytosterols/stanols in individuals with similar hypercholesterolaemia(Reference Houston, Fazio and Chilton1).
As the data on pea and lupin are scarce, the mechanism of the hypocholesterolaemic activity of plant protein may be deduced from what is known on soyabeans. Rodent and in vitro studies have established a link between the hypocholesterolaemic effects of soya and the activation/depression of liver LDL receptors(Reference Sirtori, Galli and Anderson39, Reference Lovati, Manzoni and Gianazza40). Animals on cholesterol/cholic acid dietary regimens with casein have a dramatic down-regulation of liver LDL receptors, and this effect is reversed in the presence of soya proteins. Two clinical studies have confirmed these experimental outcomes. In the former(Reference Lovati, Manzoni and Canavesi41), familial hypercholesterolaemia patients were treated with animal protein or textured soya protein (with the addition of cholesterol to balance the two diets) and both plasma lipids and LDL degradation by circulating lymphomonocytes (used as mirror images of hepatocytes) were monitored. After the animal protein diet, there were minimal changes in LDL-C levels or LDL receptor activity, whereas during the soya protein diet, in addition to a marked LDL-C reduction, an increase of approximately eightfold in LDL degradation was observed. This study was subsequently confirmed in individuals with lesser cholesterol elevations(Reference Baum, Teng and Erdman42). The bioactive soya components are probably peptides released during protein digestions(Reference Cho, Juillerat and Lee43).
The main mechanism of action of soluble fibres, instead, is linked to impaired cholesterol and bile acid absorption from the gut. A study(Reference Naumann, van Rees and Onning44) on β-glucan from oat, for example, has shown a significant reduction of two markers of cholesterol absorption (serum plant sterol concentrations) and synthesis (serum concentrations of lathosterol) by 13 and 11 %, respectively. Other mechanisms are related to the fermentation of dietary fiber in the colon by intestinal bacteria(Reference Aleixandre and Miguel45). This fermentation produces, among other products, short-chain acids (mainly acetate and propionate), which are adsorbed in the colon mucosa together with water and enter liver, where they affect lipid metabolism, including cholesterol(Reference Cani and Delzenne46).
The main mechanisms at the base of the fibre and plant protein activities being so different, it is at present difficult to hypothesise how they participate to the observed activity.
In conclusion, although this is only a first study carried out in a single centre, its successful outcomes, particularly in relation to the combination of pea and apple pectin, encourage further investigations with the participation of more centres from different nations and the use of well-designed foods with improved sensory properties for an optimal compliance. Although the market of functional foods and dietary supplements already offers numerous products for controlling cholesterol, the use of bioactive ingredient combinations opens new possibilities of choice for patients compelled to follow restricted diets for a very long period.
Acknowledgements
C. R. S. designed the clinical study, participated in the interpretation of the results and wrote a part of the manuscript; M. T., A. B., G. M. and F. P. were responsible of the patient follow-up and care; R. B. selected the participants and instructed them on the run-in and treatment diets; L. C. wrote a part of the manuscript, supervised the biological and statistical analyses and participated in the interpretation of results; V. D. V. and M. G. performed the biological analyses; C. Z. designed and prepared the dietary bars and was responsible for their blind labelling; A. A. conceived the study, wrote the proposal for getting the grant, participated in the design of the clinical study and wrote a part of the manuscript. The research was supported by a grant from the European Commission: Project “Bioprofibre” COOP-CT-2006-032075. The authors declare no conflict of interest.










