Interactions among the branched-chain amino acids (BCAA), such as the performance depressing effects of excess dietary leucine (Leu), are known in several species(Reference Harper, Miller and Block1). The impact of high dietary Leu levels needs to be elucidated in order to make correct estimates of adequate supplies and requirements for isoleucine (Ile) and valine (Val). Interactions among the BCAA include their catabolism, because all three compete for the same enzymes that catalyse the first two catabolic steps. The first step is a reversible transamination catalysed by the branched-chain amino acid transaminase (BCAT) isoenzymes, yielding branched-chain α-keto-acids (BCKA) that, in the second step, are oxidatively decarboxylated by a mitochondrial, multienzyme branched-chain α-keto acid dehydrogenase (BCKDH) complex. This step is irreversible, highly regulated and rate limiting for BCAA catabolism. The BCKDH complex consists of three catalytic components. The E1 subunit, a heterotetramer of α and β subunits, is a branched-chain α-keto acid decarboxylase. The E2 subunit is a dihydrolipoamide acyltransferase and the E3 subunit is a dihydrolipoamide dehydrogenase(Reference Yeaman2). In contrast to the other subunits, the E3 is not BCKDH-specific and its expression is not analysed in the present work. BCKDH complex activity is regulated by covalent modification. Phosphorylation of its E1α subunits by a specific BCKDH kinase (BDKDK) causes inactivation, and dephosphorylation by a specific phosphatase causes reactivation(Reference Damuni, Merryfield and Humphreys3, Reference Shimomoura, Nanaumi and Suzuki4). The abundance of the bound kinase corresponds to kinase activity(Reference Kuzuya, Katano and Nakano5). This regulation is unusual among amino acid-degrading enzymes, but is similar to the regulation of pyruvate dehydrogenase complex(Reference Wieland6). Thus, high dietary Leu levels might increase the catabolism of all BCAA and the nutritional need for Ile and Val. Additionally, there is some evidence that the growth-depressive effects of amino acid deficiencies might be caused in part by impaired action of growth hormone–insulin-like growth factor-1 (GH–IGF-1) axis(Reference Sanderson and Naik7). If excess Leu increases Ile and Val catabolism, these amino acids might become deficient and alter GH–IGF-1 expression.
Most experiments on excess dietary Leu in pigs have concentrated on performance parameters(Reference Henry, Duée and Rérat8–Reference Taylor, Cole and Lewis10), so data on metabolites, BCAA catabolism and gene expression are sparse or absent. For correct diet estimates, and to guarantee sufficient supplies of Ile and Val, data on the effects of excess dietary Leu on nutritive status are needed for weaned pigs. The aim of the present work was to determine whether excess dietary Leu affects performance, plasma amino acids, serum BCKA, activity of the BCKDH complex, mRNA levels of enzymes involved in BCAA catabolism and mRNA levels of genes related to GH–IGF-1.
Materials and methods
Animal housing and environmental conditions
Trials were conducted under supervision of the veterinary office of the Bavarian government. Animal handling and care were in accordance with the German laws for animal protection. To determine the effects of increasing dietary Leu supply to pigs fed diets without surpluses of either Ile or Val, two growth assays were conducted, each with forty-eight crossbred pigs (German Landrace × Piétrain). The pigs were raised at the same commercial plant and had an average age of 28 (sd 1) d. The initial body weight was 8·93 (sd 0·79) kg (expt 1) and 8·40 (sd 0·91) kg (expt 2). The ratio between castrated male and female pigs was balanced. Pigs were individually housed in pens (60 × 100 cm, plastic slats) in an environmentally controlled building. During the experimental period of 34 d, the room temperature was incrementally reduced from 29°C at the beginning to 25°C by the end of the experiment. Full light was provided from 07.00 to 17.00 hours, followed by half-light. Animals had ad libitum access to food and water. Animals and feeders were monitored twice daily.
Diets and experimental design
Pigs were allotted to one of six dietary treatments on the basis of their weight. Sex and ancestry were equalised across treatments in a randomised block design. Basal diets were mainly based on barley and wheat (Table 1). Maize gluten feed and soyabean meal were used as protein sources. Na-l-glutamate was included to enable the composition of isonitrogenous diets (analysed crude protein: 17·99–18·36 % in expt 1; 17·67–18·31 % in expt 2). The energy content was kept constant at 13·5 MJ metabolisable energy/kg (calculated). Diets were supplemented with essential crystalline amino acids (other than the BCAA) to meet ideal protein conditions(Reference Chung and Baker11). Tryptophan supplementation was increased to a standardised ileal digestible (SID) tryptophan:lysine ratio of 22 %. Analysed SID lysine levels ranged from 1·12 to 1·14 % in expt 1, and from 1·11 to 1·12 % in expt 2. Levels of SID amino acids in mixed diets were calculated by multiplication of the analysed amino acid contents of each feed ingredient with the estimates of standardised ileal amino acid digestibility(Reference Sauvant, Perez and Tran12). These estimates consider basal endogenous amino acid losses and are calculated as follows(Reference Stein, Sève, Fuller, Moughan and de Lange13):
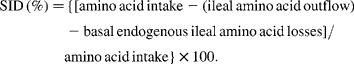
Table 1 Composition of experimental diets used in expt 1 and expt 2

MGF, maize gluten feed; SBM, soyabean meal; SGBM, sugarbeet molasses; V+M, vitamin and mineral mixture; CP, crude protein.
* Per kilogram of diet: retinol, 1·5 mg; cholecalciferol, 0·13 mg; dl-α-tocopheryl acetate, 30 mg; menadione, 150 μg; thiamin, 3 mg; riboflavin, 3 mg; pyridoxine, 3 mg; cyanocobalamin, 18 μg; nicotinic acid, 25 mg; pantothenic acid, 15 mg; biotin, 50 μg; folic acid, 300 μg; choline (as choline chloride), 300 mg; Ca, 4·9 g; P, 1·4 g; Na, 0·75 g; Mg, 0·3 g; Zn (zinc oxide), 70 mg; Fe (ferrous sulphate monohydrate), 100 mg; iodine (calcium iodate), 1·2 mg; Se (sodium selenite pentahydrate), 0·26 mg; Cu (copper sulphate pentahydrate), 10·0 mg; Mn (manganese oxide), 40·0 mg.
All diets were supplemented with vitamins and minerals to meet or exceed the requirements for 10–20 kg pigs(14).
In expt 1, the effects of increasing the dietary Leu supply in pigs fed diets with a constant Ile supply of 5·0 g SID Ile/kg of diet were estimated. Dietary Leu level of the basal group was 11·5 g SID Leu/kg and was considered as 100 % (treatment: L100). In treatments L150, L175 and L200, the Leu supply was then increased to give dietary Leu levels of 150, 175 and 200 % relative to treatment L100. Ratios of SID Leu:Ile were 2·33, 3·43, 3·98 and 4·49 in treatments L100, L150, L175 and L200, respectively. In treatment L+V 200, the Leu and Val supplies were doubled (relative to treatment L100) to determine the effects of a simultaneous excess of Leu and Val. With the last treatment, it should be tested whether the chosen Ile level of 5·0 g SID Ile/kg was first limiting and surpluses of Ile were avoided, which is a precondition to get meaningful Ile:Leu ratios. Diet composition of this positive control (PC) was equal to the basal treatment L100, but the Ile supply was increased to 6·3 g SID Ile/kg.
In expt 2, the effects of increasing the dietary Leu supply in pigs fed diets with a constant Val level of 6·2 g SID Val/kg of diet were estimated. In the basal treatment, the Leu supply was 11·1 g SID Leu/kg and was considered as 100 % (treatment L100). In the following treatments, the Leu supply was increased by l-Leu supplementation to give 150 (L150), 175 (L175) and 200 % (L200) of the Leu level of treatment L100. SID Leu:Val ratios were 1·79, 2·63, 3·08 and 3·55 in treatments L100, L150, L175 and L200, respectively. In treatment L+I 200, the Leu and Ile supplies were doubled (relative to treatment L100) to determine the effects of a simultaneous excess of Leu and Ile. Again, the last treatment was a PC. Diet composition was equal to treatment L100, but the Val supply was increased to 7·3 g SID Val/kg.
Sampling
Trials were terminated on day 34, when the pigs were fasted for 2·5 h. Afterwards, two blood samples per pig were taken via jugular vein puncture. The first sample was used to determine plasma-free amino acids (9 ml, S-Monovette, Li-Heparin, Sarstedt, Nümbrecht, Germany). The second sample was used to determine serum BCKA (9 ml, Gel-S-Monovette, Sarstedt, Nümbrecht, Germany). The samples were cooled by ice water. Plasma and serum were obtained by centrifugation (20 min, 800 g), and stored at − 80°C.
After blood sampling, the pigs remained for 3 d at the experimental piggery, where the feeding diet regimen was switched to three meals per day. The daily amount of feed was calculated for each individual pig by multiplication of its metabolic body weight with a treatment-specific factor of average daily feed intake per kilogram metabolic body weight, estimated for the last experimental week. On day 38, all pigs of expt 1+2 undergoing treatments L100, L150 and L200 were killed by captive bolt pistol and exsanguinated by transection of the carotid arteries in randomised order. All pigs were fed 1/3 of their daily feed amount at 07.00 hours. The first pig was killed at 09.30 hours. For every delay, the following pigs received an extra meal (1/24 of the daily feed amount per h of delay), at 2·5 h before killing, to ensure comparable postprandial conditions. After exsanguinations, samples of skeletal muscle (musculus longissimus dorsi, seventh rib), heart, liver, spleen, jejunum and ileum (mid parts) were removed and quick frozen in liquid nitrogen until storage at − 80°C. Response variables were daily gain, daily feed intake, feed efficiency, plasma amino acids, serum BCKA, the BCKDH activity in liver samples, mRNA of genes encoding enzymes in BCAA catabolism and mRNA of genes related to GH–IGF-1.
Feed analyses
Crude protein content was determined using the Kjeldahl procedure(Reference Naumann and Bassler15). Analysis of dietary amino acids was carried out by Ajinomoto Eurolysine S.A.S (Paris, France). Amino acid contents were analysed by ion exchange chromatography after acid hydrolysis with HCl (6 m, reflux for 23 h at 110°C)(16). Methionine and cysteine were assayed after performic acid oxidation(16). Tryptophan was determined by reversed-phase HPLC and fluorometric detection after alkaline hydrolysis with barium hydroxide (16 h, 120°C)(17).
Plasma-free amino acids
Plasma proteins were removed by precipitation with salicyl-sulphonic acid and centrifugation (11 000 g, 10 min). After dilution with a lithium acetate solution, the protein-free supernatant was analysed by ion exchange chromatography on an automatic amino acid analyser (LC 3000, Biotronik, Hamburg, Germany)(Reference Naumann and Bassler15).
Branched-chain α-keto-acids determination
BCKA were analysed as reported by Pailla et al. (Reference Pailla, Blonde-Cynober and Aussel18). BCKA were derivatised with o-phenylenediamine to give fluorescent derivates, which were separated chromatographically on a reversed-phase column (Spherisorb ODS-2, 4·6 × 250 mm, 5 μm particles, Waters, Eschborn, Germany) using a binary gradient (L-7100, Merck-Hitachi, Tokyo, Japan). Detection was performed fluorometrically (FL Detector L-7480, Merck-Hitachi). α-keto-valerate was used as an internal standard.
Branched-chain α-keto acid dehydrogenase activity
BCKDH activity was assayed spectrophotometrically(Reference Nakai, Kobayashi and Popov19). A frozen tissue sample was pulverised to a fine powder under liquid nitrogen. Thereafter, 0·25 g of the powder was homogenised (motor driven Tefflon pestle) in ice-cold extraction buffer. Insoluble material was removed by centrifugation (20 000 g, 5 min, 4°C), and the supernatant was made 9 % (v/v) in polyethylene glycol. After 20 min on ice, a second centrifugation step (12 000 g, 10 min, 4°C) was performed, and the pellet dissolved in suspension buffer. BCKDH activity was determined at 30°C using α-keto-isovalerate (KIV) as substrate, by measuring absorbance at 340 nm to detect NADH formation. To determine total BCKDH activity, the tissue extract was incubated (20 min, 37°C) with λ protein phosphatase before measurement. In contrast to Nakai et al. (Reference Nakai, Kobayashi and Popov19), the assay buffer was made without dihydrolipoamide dehydrogenase.
Total RNA extraction
Total RNA was extracted with peqGOLD TriFast (PEQLAB Biotechnologie, Erlangen, Germany) that uses a one-step liquid-phase separation using phenol and guanidine isothiocyanate in a single liquid phase. Tissue samples (50 mg) were homogenised using the MagNA Lyser Green beads (Roche Diagnostics, Mannheim, Germany) in the presence of peqGOLD TriFast (0·5 ml). After addition of chloroform (100 μl) and centrifugation (12 000 g, 15 min), the homogenate was separated into three phases, with the RNA in the upper aqueous phase. The extracted RNA, free of DNA and proteins, was precipitated with isopropanol (150 μl) and centrifugation (12 000 g, 10 min, 4°C). Pellets were washed twice with 250 μl of 75 % (v/v) ethanol at − 20°C and centrifugation (10 000 g, 5 min, 4°C). RNA was dissolved in 30 μl diethyl pyrocarbonate-treated water and stored at − 80°C.
Liver samples were additionally treated with peqGOLD OptiPure (PEQLAB Biotechnologie) to eliminate polysaccharides. Pellets obtained by extraction with peqGOLD TriFast were mixed with 100 μl peqGOLD OptiPure. After centrifugation (3000 g, 10 min, 4°C), the supernatant was removed and 100 μl SDS (0·5 % (w/v), pH 7) was added. After incubation for 5 min at 55°C, 100 μl chloroform was added and the mixture was centrifuged (3000 g, 5 min, 4°C). Natrium acetate (2 m, pH 5) was added to the supernatant to a final concentration of 0·2 m. RNA was precipitated with isopropanol (100 μl). Centrifugation and washing steps were as described for the extraction. Pellets were dissolved in 60 μl diethyl pyrocarbonate-treated water.
RNA quantity was determined photometrically (Nanodrop 1000, PEQLAB Biotechnologie) and purity calculated from the 260/280 nm absorbance ratio. All working solutions were diluted to a RNA concentration of 10 ng/μl.
RNA quality
Integrity of RNA was analysed for six random samples per tissue and trial using the RNA 6000 Nano assay (Agilent Technology, Palo Alto, CA, USA) and the 2100 Bioanalyser (Agilent Technology). The RNA integrity number served as an RNA quality parameter ranging from one (the most degraded profile) to ten (the most intact profile).
Primer design
Primers were designed using published RNA sequences of pigs (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi), if available. Otherwise, they were designed as nested primers from homologous regions of cattle, human and rat genes. Primer design accounted for primer–dimer formation, self-priming and primer annealing temperature (60°C) (http://fokker.wi.mit.edu/primer3/input.htm). Primers were synthesised by Eurofins MWG Operon (Ebersberg, Germany) and are listed in Table 2. Primer testing included different annealing temperatures and product validation via agarose gel electrophoresis for four random samples and a negative control for each primer set.
Table 2 Sequences of primers used for quantitative real time-PCR and product sizes

GAPDH, glyceraldehyde- 3-phosphate dehydrogenase; BCATm, mitochondrial branched-chain amino transferase; BCKDH E1α, branched-chain α-keto acid dehydrogenase E1α subunit; BCKDK, branched-chain α-keto acid dehydrogenase kinase; 4-HPPD, 4-hydroxyphenylpyruvate dioxygenase; GHR, growth hormone receptor; IGFBP-3, insulin-like growth factor-binding protein 3; IGFALS, insulin-like growth factor acid labile subunit; IGFR, insulin-like growth factor receptor; IGF-1, insulin-like growth factor 1.
PCR analysis
Quantitative real time PCR was conducted using a SuperScript III Platinum SYBR Green One-Step quantitative real time PCR Kit (Invitrogen Corporation, Carlsbad, CA, USA). For each sample, 5 μl 2X SYBR Green Reaction Mix, 0·5 μl forward primer (10 μm), 0·5 μl reverse primer (10 μm) and 0·2 μl SuperScript III RT/Platinum Taq Mix were mixed, and 3·8 μl template (total RNA concentration: 10 ng/μl) was added. quantitative real time PCR was performed with a Rotor-Gene 6000 (Analysis Software 6.0; Corbett Life Science, Sydney, NSW, Australia) using the following protocol: hold step (55°C, 3 min); denaturation step (95°C, 5 min); cycling (95°C, 15 s; 60°C, 10 s; 68°C, 20 s; 40 cycles); hold step (40°C, 1 min); melting curve analysis.
The products obtained by quantitative real time PCR were validated by 1·8 % agarose gel electrophoresis at 90 V for 30 min. After gel extraction (Wizard SV Gel and PCR Clean-Up system, Promega Cooperation, Madison, WI, USA), sequencing was performed by the Department Biology Genomics Service unit at the Ludwig-Maximilians-Universität München. Nested primers were designed from the determined sequences.
Statistical analyses
Data were analysed by ANOVA using the mixed procedure of Statistical Analysis Systems statistical software package version 9.1. (SAS Institute, Cary, NC, USA), with individual pigs as experimental units. The model included treatment and sex as fixed and litter as random effects. Initial body weight was used as a covariate. Contrasts were performed to determine the linear and quadratic effects of an increasing dietary Leu supply(Reference Lowry20). The integrated matrix language procedure was used to generate orthogonal polynomial coefficients, and the mixed procedure was used to compute orthogonal polynomial sums of squares. P-values for treatment are given in the tables. The effects of doubling the Val or Ile supply at high levels of dietary Leu were tested for significance using the Tukey adjustment (expt 1: L200 v. L+V 200; expt 2: L200 v. L+I 200). The effects of the PC were tested for significance using the Tukey adjustment (L100 v. PC).
mRNA expression data were analysed using the relative quantification method that describes the change in target gene expression relative to the control group. β-actin, ubiquitin and glyceraldehyde-3-phosphate dehydrogenase genes were used as normalising internal controls for the amount of RNA added to reverse transcription reactions. Internal control gene expression was analysed for every tissue and every sample. For normalisation, the tissue- and sample-specific arithmetic means of the three internal control genes served as a control gene index. Cycle thresholds of the target genes were subtracted from the control gene index (ΔC t) for normalisation, and treatment groups were compared to the control group (ΔΔC t;(Reference Livak and Schmittgen21)), using the Statistical Analysis Systems general linear models procedure and the Tukey adjustment. To determine gene expression differences between tissues, the Statistical Analysis Systems general linear models procedure and the Tukey adjustment were applied to C t values.
Results
Performance
In expt 1, increasing the dietary Leu supply from treatment L100 to L200 linearly decreased the daily feed intake (P = 0·06) and gain (P = 0·05) by 14–15 % (Table 3). A regression for the average daily gain (y) as a function of the SID Leu:Ile ratio (x) was estimated as y = 548·5 − 34·2x (R 2 0·971; P = 0·02). In contrast, no major impact was seen on feed efficiency. Doubling the Val supply at high dietary levels of Leu did not affect the performance (L+V 200 v. L200; P>0·05). The growth performance of PC was not different from that of L100 (P>0·05).
Table 3 Effects of increasing leucine supply and of simultaneous leucine and valine excess on the performance of weaned pigs fed diets first limiting in isoleucine (expt 1; n 8)*
(Mean values with their standard errors)
SID, standardised ileal digestible; IBW, initial body weight; FBW, final body weight; FI, feed intake; G:F, gain to feed.
* For details of animals and procedures, see Materials and methods.
† Polynomial orthogonal contrasts were estimated for L100–L200.
In expt 2, incrementally increasing Leu supply from L100 to L200 linearly decreased the performance parameter in a dose-dependent manner (P < 0·01; Table 4). The daily feed intake, daily gain and feed efficiency decreased by 30, 38 and 11 %, respectively. A regression for the average daily gain (y) as a function of the SID Leu:Val ratio (x) was estimated as y = 538·4 − 84·7x (R 2 0·955; P = 0·02). In treatment L+I 200, doubling the Ile supply did not affect the performance relative to L200. In PC, increasing the Val supply significantly (P < 0·05) increased the animals performance. Compared to L100, the daily feed intake and gain of PC were increased by 38 and 43 %, respectively.
Table 4 Effects of increasing leucine supply and of simultaneous leucine and isoleucine excess on the performance of weaned pigs fed diets first limiting in valine (expt 2; n 8)†
(Mean values with their standard errors)

SID, standardised ileal digestible; IBW, initial body weight; FBW, final body weight; FI, feed intake; G:F, gain to feed.
Mean values were significantly different from those of the L100 group: *P < 0·05.
† For details of animals and procedures, see Materials and methods.
‡ Polynomial orthogonal contrasts were estimated for L100 to L200.
Plasma metabolites
In expt 1, increasing the dietary Leu supply increased plasma Leu levels linearly (P < 0·01) and quadratically (P = 0·03), and the serum α-keto-isocaproate (KIC) levels linearly (P < 0·01), in a dose-dependent manner (Table 5). Doubling the Leu supply increased plasma Leu levels by 64 % and serum KIC levels by 45 %. Plasma levels of histidine, threonine and serine increased linearly (P < 0·01), and plasma methionine and proline were influenced quadratically (P = 0·03). In contrast, plasma Val and serum KIV levels decreased linearly (P < 0·01) and quadratically (P < 0·01) up to 60 and 72 %, respectively. However, the plasma Ile and serum α-keto β-methylvalerate (KMV) levels remained unaffected by increasing Leu supply (P>0·05).
Table 5 Effects of increasing leucine supply and of simultaneous leucine and isoleucine excess on plasma-free amino acids and serum branched-chain α-keto-acids of weaned pigs fed diets first limiting in isoleucine (expt 1; n 8)‡
(Mean values with their standard errors)

SID, standardised ileal digestible; ΣEAS, sum of the essential amino acids; ΣNEAS, sum of the non-essential amino acids; KMV, α-keto-β-methylvalerate; KIC, α-keto-isocaproate; KIV, α-keto-isovalerate.
Mean values were significantly different from those of the L200 group: *P < 0·05.
Mean values were significantly different from those of the L100 group: †P < 0·05.
‡ For details of animals and procedures, see Materials and methods.
§ Polynomial orthogonal contrasts were estimated for L100 to L200.
Doubling the dietary Val supply at high dietary levels of Leu in L+V 200 increased plasma Val levels 4-fold (P < 0·05) and serum KIV levels 5·8-fold (P < 0·05), but decreased plasma glutamic acid by 32 % (P < 0·05) compared to L200.
Compared to L100, increasing the dietary Ile supply in PC significantly (P < 0·05) increased the plasma Ile and serum KMV levels 4·6- and 5-fold, respectively. In contrast, serum KIV levels decreased by 26 % (P < 0·05).
In expt 2, increasing the dietary supply of Leu increased plasma Leu linearly (P < 0·01) and serum KIC linearly (P < 0·01) and quadratically (P = 0·04; Table 6). Doubling the dietary Leu supply increased the plasma Leu and serum KIC levels by 59 and 49 %, respectively. In contrast, plasma Ile and serum KMV levels decreased linearly (P < 0·01) and quadratically (P = 0·03) up to 69 and 66 %, respectively. Plasma levels of aspartic acid (P < 0·01) and glutamine (P < 0·05) also decreased linearly, whereas histidine (P < 0·01) and phenylalanine (P < 0·05) increased linearly (data not shown). Plasma Val levels were influenced quadratically (P = 0·03) and serum KIV levels increased linearly (P < 0·05).
Table 6 Effects of increasing leucine supply and of simultaneous leucine and isoleucine excess on plasma-free amino acids and serum branched-chain α-keto-acids of weaned pigs fed diets first limiting in valine (expt 2; n 8)‡
(Mean values with their sandard errors)

SID, standardised ileal digestible; ΣEAS, sum of the essential amino acids; ΣNEAS, sum of the non-essential amino acids; KMV, α-keto-β-methylvalerate; KIC, α-keto-isocaproate; KIV, α-keto-isovalerate.
Mean values were significantly different from those of the L200 group: *P < 0·05.
Mean values were significantly different from those of the L100 group: †P < 0·05.
‡ For details of animals and procedures, see Materials and methods.
§ Polynomial orthogonal contrasts were estimated for L100 to L200.
Doubling the dietary Ile supply at high dietary Leu levels increased the plasma Ile and serum KMV levels 2·9- and 2·4-fold (P < 0·05), respectively (L200 v. L+I 200).
Increasing the dietary Val supply in the PC significantly (P < 0·05) increased the plasma Val and serum KIV levels 5·6- and 13·6-fold, respectively, compared to L100. Plasma cysteine levels also increased (P < 0·05). In contrast, plasma methionine, phenylalanine, threonine, alanine, asparagine, glycine and serine levels decreased (P < 0·05; data not shown).
Branched-chain α-keto acid dehydrogenase activities in the liver
In expt 1, the total BCKDH activity was not altered by increasing Leu supply (Fig. 1(a)). In contrast, the basal activity (Fig. 1(c)) and the level of activation (Fig. 1(e)) increased linearly (P < 0·01) by 165 and 135 %, respectively.

Fig. 1 Effects of increasing leucine supply on branched-chain α-keto acid dehydrogenase (BCKDH). Total ((a) and (b)), basal ((c) and (d)) and relative ((e) and (f)) BCKDH activities in the liver of pigs fed diets first limiting in isoleucine (expt 1) or valine (expt 2). For (e), y = − 7·5+8·9x and for (f), y = − 4·2+7·2x. Values are means with their standard errors depicted by vertical bars (n 8). a,b Mean values within a graph with unlike superscript letters were significantly different (P < 0·05).
In expt 2, increasing the dietary Leu supply did not affect the total BCKDH activity (Fig. 1(b)). In contrast, the basal activity (Fig. 1(d)) and the corresponding level of activation (Fig. 1(f)) increased linearly (P < 0·01) in a dose-dependent manner as the Leu supply increased. Increasing the SID Leu:Val ratio from 1·79 to 3·55 increased the basal BCKDH activity and its level of activation approximately 3-fold.
mRNA expression
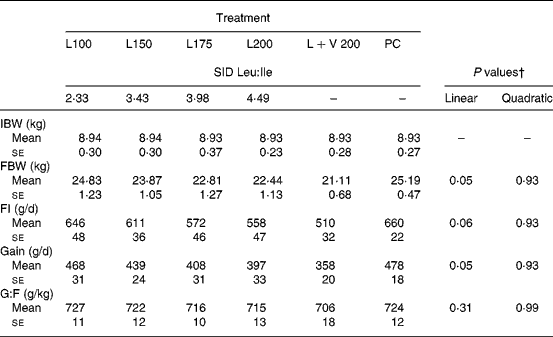
The mean RNA integrity number value was 7·91 (sd 0·14), documenting an integer total RNA. For both expt 1 and expt 2, the internal control genes were unaffected by the experimental treatment (P>0·05). In expt 1, increasing the dietary Leu supply did not affect the expression of the mitochondrial BCAT (BCATm), BCKDH kinase (BCKDK) or BCKDH subunit (E1α, E1β and E2) genes in skeletal muscle, heart, spleen, jejunum and ileum (data not shown). In liver tissue, however, the mRNA levels of genes for BCKDH E1β and BCKDK were significantly altered (P < 0·05; Table 7). Compared to L200, mRNA of the BCKDH E1β gene was significantly lower in L150 but unchanged in L100. In contrast, the mRNA levels of the BCKDK gene increased in L200.
Table 7 Effects of increasing leucine supply on mRNA of branched-chain amino acid catabolism genes in the liver of weaned pigs fed diets first limiting in isoleucine (expt 1)*
(Mean values with their standard errors)

BCKDH, branched-chain α-keto acid dehydrogenase; SID, standardised ileal digestible; BCATm, mitochondrial branched-chain amino acid transferase; BCKDK, branched-chain α-keto acid dehydrogenase kinase; 4-HPPD, 4-hydroxyphenylpyruvate dioxygenase.
a,b Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* 2− ΔΔC t values; for details, see Materials and methods.
Tissue-specific differences were observed in the mRNA expressions of the catabolic enzymes (Table 8), and are summarised as follows:
BCATm, heart>>skeletal muscle, spleen, jejunum>>ileum, liver;
BCKDH E1α, heart>>liver>>jejunum, skeletal muscle>>spleen, ileum;
BCKDH E1β, heart>>liver>>jejunum>>skeletal muscle, spleen>>ileum;
BCKDH E2, heart, liver>>ileum, spleen, jejunum>>skeletal muscle;
BCKDK, heart>>skeletal muscle>>liver>jejunum>ileum>spleen.
Table 8 Tissue-specific take-off values estimated by quantitative real time PCR for expt 1*
(Mean values with their standard errors)
LD, longissimus dorsi; BCATm, mitochondrial branched-chain amino acid transferase; BCKDH, branched-chain α-keto acid dehydrogenase; BCKDK, branched-chain α-keto acid dehydrogenase kinase.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of animals and procedures, see Materials and methods.
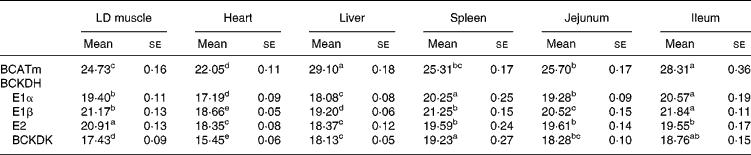
In expt 2, no significant alterations were seen in mRNA expressions of the genes for BCATm, BCKDH subunits and BCKDK neither in muscle nor in liver tissue (data not shown). The mRNA expression of 4-hydroxyphenylpyruvate dioxygenase (4-HPPD) genes in liver and muscle tissue was neither influenced (P>0·05) in expt 1 nor in expt 2 (data not shown). Increasing dietary Leu supply did not affect (P>0·05) the mRNA expressions of the genes for growth hormone receptor (GHR), IGFBP, IGF acid labile subunit (IGFALS), IGF receptor and IGF-1 in skeletal muscle and liver tissue of expt 1, or in skeletal muscle of expt 2 (data not shown). However, in liver tissue, in expt 2, mRNA from the genes for GHR, IGFALS and IGF-1 was significantly decreased (P < 0·05; Table 9).
Table 9 Effects of increasing dietary leucine supplementation on mRNA of genes related to growth hormone–insulin-like growth factor-1 axis in the liver of pigs fed diets first limiting in valine (expt 2)*
(Mean values with their standard errors)
SID, standardised ileal digestible; GHR, growth hormone receptor; IGFBP-3, insulin-like growth factor-binding protein 3; IGFALS, insulin-like growth factor acid labile subunit; IGFR, insulin-like growth factor receptor; IGF-1, insulin-like growth factor-1.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* 2− ΔΔC t values; for details, see Materials and methods.
Discussion
In the present work, the effects of increasing dietary Leu levels in isonitrogenous diets were determined. Surpluses of Ile (expt 1) or Val (expt 2) were avoided to get meaningful ratios to Leu and to increase the animals' sensitivity for interactions between the BCAA. In L100, the Ile supply was set at 5·0 g SID Ile/kg diet (SID Leu:Ile ratio of 2·33). Incremental supplementation with Leu decreased the daily feed intake and gain in a dose-dependent manner. Increasing the SID Leu:Ile ratio from 2·33 to 4·49 (L100 to L200) decreased the daily feed intake and gain by 14 and 15 %, respectively.
In expt 2, the Val supply was set at 6·2 g SID Val/kg diet. Incremental Leu supplementation (SID Leu:Val ratio from 1·79 to 3·55; L100 to L200) decreased the daily feed intake, daily gain and feed efficiency linearly, in a dose-dependent manner, by 30, 38 and 11 %, respectively. In the PC, the Val supply was increased to 7·3 g SID Val/kg diet, which increased the daily feed intake and gain, indicating suboptimal Val supply.
These findings are in accordance with the recent literature. Langer & Fuller(Reference Langer and Fuller22) showed that excess dietary Leu decreases the nitrogen utilisation of pigs fed diets marginal for Ile or Val by 12–18 %. In earlier works, however, no effect occurred after increasing the Leu:Ile ratio from 1·7 to 3·0 in 15 − 51 kg pigs(Reference Henry, Duée and Rérat8), suggesting that increasing the Leu:Ile ratio above 3·5 may have negative effects in growing pigs(Reference Taylor, Cole and Lewis9). In contrast, we showed that excess dietary Leu linearly decreased the performance, when a SID Leu:Ile ratio of 2·33 or a SID Leu:Val ratio of 1·79 was exceeded.
In 30–40 kg pigs fed Ile-limited diets, the negative effects of excess Leu on nitrogen utilisation are reversible by a simultaneous excess of Val(Reference Langer and Fuller22). In the present work, however, no benefits were detected for doubling the supply of Ile or Val in Leu-excessive diets. In contrast to Langer & Fuller(Reference Langer and Fuller22), no significant increase in performance was observed, and the benefits of excess dietary Val in diets excessive in Leu could not be confirmed.
The basal dietary Ile and Val levels in expt 1 and 2 were chosen to be first limiting in order to guarantee that any observed negative effects of increasing dietary Leu levels would be direct and not obscured. Compared to PC, the marginality of the Ile supply in expt 1 was not reflected in the animals' performance, but was clearly demonstrated by a sharp decrease in plasma Ile and serum KMV levels. In expt 2, the marginality of the Val supply was indicated by a performance depression, and confirmed by a sharp decrease in plasma Val and serum KMV levels. The low plasma Ile and Val levels seen in the present work were in accordance with the literature(Reference Bravo, Meade and Stockland23–Reference Figueroa, Lewis and Miller25).
In both trials, increasing the dietary Leu supply significantly increased the plasma Leu and serum KIC levels. Doubling the dietary Leu supply increased the plasma Leu and serum KIC levels by 64 and 45 %, respectively, in expt 1, and by 59 and 49 %, respectively, in expt 2. However, plasma Ile and Val, and serum KMV and KIV levels decreased with increasing dietary Leu levels. These findings indicate increased BCAA catabolism in accordance with the literature. An increased catabolism of Val and Ile was seen in chicks fed diets with excess Leu(Reference Calvert, Klasing and Austic26). In growing gilts fed diets containing high levels of dietary Leu for 7 d, reduced plasma concentrations of Val, Ile and BCKA were seen(Reference Langer, Scislowski and Brown27). The same effects were reported for rats(Reference Harper and Benjamin28, Reference Block and Harper29). The negative effects of excess dietary Leu are reversed by supplementation with Ile and Val in chicks, pigs and kittens(Reference Calvert, Klasing and Austic26, Reference Fu, Fent and Allee30, Reference Hargrove, Rogers and Calvert31), confirming the hypothesis of an increased nutritional need for Ile and Val in diets with excess Leu.
The first step of the BCAA catabolism is a transamination catalysed by the BCAT, which exists as BCATm and cytosolic BCAT isoenzymes. The BCAT enzymes are widely distributed among tissues with cytosolic BCAT restricted to neural tissues. Therefore, we concentrated on BCATm. In the literature, the highest BCATm activities are reported for peripheral tissues such as skeletal muscle, whereas BCATm is not expressed in rodent and human liver(Reference Harper, Miller and Block1, Reference Suryawan, Hawes and Harris32). To our knowledge, we are the first to publish an overview about tissue-specific BCATm RNA expression in pigs. In the present work, the highest BCATm RNA expression was seen in heart and skeletal muscle, whereas the lowest expression was seen in ileum and liver. Expression of BCATm RNA in the heart increased about 137-fold compared to liver. These findings confirm the suggestion that the BCAA escape first-pass metabolism in the liver and thus are available to peripheral tissues. Increasing dietary Leu supply in the present work did not affect the mRNA levels of BCATm.
The second step in BCAA catabolism is catalysed by BCKDH. The highest BCKDH activities are reported in liver with intermediate activity in heart, kidney and brain, and low activity in skeletal muscle(Reference Harper, Miller and Block1). This is largely consistent with the mRNA levels of weaned pigs, reported for the first time in this work. The highest mRNA levels of all analysed BCKDH subunit genes (E1α, E1β and E2) were seen in heart and liver, and the lowest in skeletal muscle and ileum.
BCKDK activity is reciprocal to BCKDH activity. Therefore, decreased expression of the BCKDK gene could cause an enhanced BCKDH activity. However, a significant increase in BCKDK gene expression was seen in liver tissue at the highest Leu supply in expt 1. Nonetheless, the alterations in BCKDK gene expression were minor changes of about 20 %, and only the bound form of the kinase regulates BCKDH activity. This should be considered in assessing the informative value of BCKDK mRNA expression with regard to changes in BCKDH activity. In the present study, tissue-specific expressions of BCKDK mRNA were reported for pigs, and found to be highest in heart and skeletal muscle, and lowest in spleen and ileum. The differences between skeletal muscle and liver tissue were 1/10 of that seen in human subjects(Reference Suryawan, Hawes and Harris32).
The KIC, the corresponding BCKA of Leu, plays an important role in the regulation of BCKDH activity. High KIC levels can increase the catabolism of the other BCAA by inhibition of the BCDKH kinase. This results in a less phosphorylated and therefore more active BCKDH complex(Reference Paxton and Harris33). Block et al. (Reference Block, Aftring and Mehard34) reported that increases in plasma Leu paralleled changes in the activity of the BCKDH complex. In contrast to Leu, Val and Ile seem to be unimportant for the regulation of BCKDH activity. Infusions of Val or Ile failed to activate BCKDH in rats(Reference Aftring, Block and Buse35). In the present work, doubling the dietary Leu supply increased the serum KIC levels by 45–49 % and the basal activity of the liver BCKDH 2- to 3-fold. An increased rate of Leu oxidation was reported in rats when the dietary Leu supply exceeded the requirement for maximum rate of weight gain(Reference Harper and Benjamin28). Increased BCKDH activity was seen in rats and pigs fed diets excessive in Leu(Reference Kuzuya, Katano and Nakano5, Reference Langer, Scislowski and Brown27, Reference Block and Harper29). The levels of BCKDH activity and its activity state in the present work were in accordance with the levels seen in growing gilts fed semipurified diets excessive in Leu, but marginal in Ile or Val(Reference Langer, Scislowski and Brown27). The liver BCKDH activity states in the present work ranged from 7 to 27 % and are comparable to that of human subjects (26 %), but much smaller than that of rats (88 %)(Reference Suryawan, Hawes and Harris32). The enhanced BCKDH activity in liver seen in this work resulted from an increased grade of activity. The total BCKDH activity as well as the gene expression of the BCKDH subunits (E1α, E1β and E2) remained almost unaffected, indicating a posttranscriptional regulation.
Our findings are in accordance with Matsuzaki et al. (Reference Matsuzaki, Kato and Sakai36), who investigated the impact of increasing dietary Leu supply (up to 15 % of the diet) on gene expression in rats using DNA microarrays. The data showed small alterations (less than 2-fold) in the expression of BCAA catabolism enzyme genes. However, it was reported that feeding BCAA-enriched diets to rats increased the activity state of the BCKDH through a combination of decreased BCKDK activity and increased total BCKDH activity(Reference Kuzuya, Katano and Nakano5). The increased BCKDH catabolism reported by Kuzuya et al. (Reference Kuzuya, Katano and Nakano5) was mainly caused by an increased activity state. The total BCKDH activity increased maximally by 45 %, whereas the activity state increased 8·5-fold. In conclusion, in pigs as in rats, excess dietary Leu seemed not to alter the gene expression of enzymes involved in BCAA catabolism to a great extent. Regulatory mechanisms that adapt to excess Leu appear to implicate mainly cellular posttranscriptional mechanisms.
In the context of dietary Leu oversupply, alternative pathways for Leu catabolism, which bypass BCKDH, might be of some importance. In addition to its oxidation by BCKDH, KIC can be converted to β-hydroxy-β-methylbutyrate by 4-HPPD (identical to KIC dioxygenase)(Reference Crouch, Lee and Iturriagagoitia-Bueno37). The activity of 4-HPPD was found to be 14 % of the total BCKDH activity in human liver(Reference Xu, Nakai and Ishigure38), illustrating its importance. In expt 1 and 2, increasing dietary Leu supply had no impact on expression of the liver 4-HPPD gene. Feeding a BCAA-rich diet to rats increased BCKDH, but not 4-HPPD activity(Reference Xu, Nakai and Ishigure38). In conclusion, the effects of excess Leu on 4-HPPD require further investigation. 4-HPPD is an important enzyme of tyrosine catabolism(Reference Lindstedt, Holme and Lock39), and an impact of high KIC levels on tyrosine catabolism seems possible. However, we found no alterations in plasma tyrosine levels as the dietary Leu supply increased.
For several species, excess dietary Leu causes a growth depression(Reference Harper, Miller and Block1). The present work confirms this general effect, although the mode of action remains unexplained. The hypothesis that growth retardation is a consequence of excess dietary Leu suggests a nutritional impact on the GH–IGF-1 axis, for which the literature contains some evidence(Reference Sanderson and Naik7). Therefore, we decided to analyse the mRNA levels of GHR, IGF-binding protein 3, IGFALS, IGF receptor and IGF-1 in skeletal muscle and liver. In expt 1, no impact on genes of the GH–IGF-1 axis in skeletal muscle or liver was observed when dietary Leu levels were increased. In contrast, increasing Leu supplementation decreased the mRNA levels of GHR, IGFALS and IGF-1 genes in liver tissue in expt 2. This is consistent with the greater extent of growth retardation in expt 2, compared to expt 1. Generally, fasting decreases the expression of the GH–IGF-1 axis genes(Reference Clemmons and Underwood40, Reference Straus and Takemoto41). In the present work, fasting or anorexia did not occur, but the daily feed intake decreased linearly with the increases in Leu supplementation. The stimulation of IGF-1 secretion by GH seems to be dependent on the availability of specific amino acids. Single deletions of arginine, proline, threonine, tryptophan or Val caused a block of GH-stimulated IGF-1 gene expression in cultured pig hepatocytes(Reference Brameld, Gilmour and Buttery42). However, the present work had no essential amino acid depletion. In contrast to previous works, we showed alterations in the expression of GH–IGF-1 axis genes caused by an increasing Leu supply in a sound organism. The increased Leu supply decreased the GHR, IGFALS and IGF-1 gene expression in expt 2 but not in expt 1, indicating a more extended Leu-induced nutrient shortage for expt 2. We conclude that the greater growth retardation seen in expt 2 might be partially caused by decreased activity of the GH–IGF-1 axis as a result of Leu-induced Val deficiency.
In the present work, feed intake decreased as the dietary Leu content increased. In force-fed chicks, it has been found that about 70 % of performance depression caused by Leu oversupply was the result of a decreased feed intake(Reference Calvert, Klasing and Austic43). It is known from preference trials that rats reject diets excessive in Leu and instead prefer an alternative diet even if it is protein free(Reference Rogers, Tannous and Harper44). It has been shown by injection of l-Leu into the brain of rats that dietary Leu is indeed a nutrient signal, which can cause depressions of feed intake(Reference Cota, Proulx and Blake Smith45, Reference Ropelle, Pauli and Fernandes46). Leu activates the hypothalamic mammalian target of rapamycin pathway and decreases the adenosine monophosphate-activated protein kinase activity, which results in inhibition of neuropeptide Y and stimulation of pro-opiomelanocortin expression(Reference Cota, Proulx and Blake Smith45, Reference Ropelle, Pauli and Fernandes46). Neuropeptide Y promotes feeding, decreases energy expenditure and silences pro-opiomelanocortin, whereas pro-opiomelanocortin promotes satiety(Reference Gao and Horvath47). Therefore, inhibition of neuropeptide Y and activation of pro-opiomelanocortin cause satiety and give an explanation for the Leu-induced anorexia. Inhibition of mammalian target of rapamycin with rapamycin inhibited the l-Leu-induced anorexia(Reference Cota, Proulx and Blake Smith45).
In conclusion, growth depression as a consequence of excess dietary Leu is accompanied by decreased plasma levels of Ile and Val, and increased BCKDH activities, indicating an increase in BCAA catabolism. Regulation of BCAA catabolism mainly involved posttranscriptional mechanisms, because no major alterations were seen in gene expression of BCATm, BCKDH subunits or BCKDK. The alterations seen in the mRNA of GHR, IGFALS and IGF-1 in pigs fed Leu-excessive diets need further investigation.
Acknowledgements
Funding for the present project was provided by Arbeitskreis für Tierernährungsforschung Weihenstephan (ATW) e.V. Realisation of experimental and laboratorial work as well as writing was done by M. K. W. M. W. P. made mRNA expression analyses possible and provided laboratory facilities and assistance. Experimental design and conception, acquisition of financial resources and proof reading was done by F. X. R. There are no conflicts of interest.














