Anxiety and depression are among the most common mental disorders in the United States (Anxiety and Depression Association of America, 2016; National Institute of Mental Health, 2016), and among the most prevalent causes of disability worldwide (World Health Organization, 2017). Hence, it is critical to improve current understanding of the neurobehavioral mechanisms associated with functions that are altered in these conditions. Despite recent progress regarding specific brain regions and personality traits (e.g., DeYoung et al., Reference DeYoung, Hirsh, Shane, Papademetris, Rajeevan and Gray2010; Giuliani, Drabant, Bhatnagar, & Gross, Reference Giuliani, Drabant, Bhatnagar and Gross2011), it remains unclear whether there are common latent brain and personality factors that might predict resilience or vulnerability to emotional distress.
Volumetric alterations in the brain and individual differences in personality traits that support resilience to emotional distress have been consistently associated with anxiety and depression (Chang et al., Reference Chang, Yu, McQuoid, Messer, Taylor, Singh and Payne2011; Gross & John, Reference Gross and John2003; Martin & Dahlen, Reference Martin and Dahlen2005; Talati, Pantazatos, Schneier, Weissman, & Hirsch, Reference Talati, Pantazatos, Schneier, Weissman and Hirsch2013; van Tol et al., Reference van Tol, van der Wee, van den Heuvel, Nielen, Demenescu, Aleman and Veltman2010; Watson, Clark, & Carey, Reference Watson, Clark and Carey1988; Wu et al., Reference Wu, Feder, Cohen, Kim, Calderon, Charney and Mathe2013). However, it remains unclear how these factors are interrelated, and whether there are common brain and personality factors underlying these associations. Examining this issue using integrative models combining neural correlates, personality traits, and measures indexing symptoms of distress in healthy populations provides the opportunity to identify common individual difference factors associated with different types of distress that may index resilience to, or risk/vulnerabilities for, psychopathologies (Cuthbert & Insel, Reference Cuthbert and Insel2013). Hence, in this proof-of-concept study, we adopted a brain-personality-distress symptom framework, using an integrative structural equation modeling approach, to examine associations among latent constructs of brain region volumes and personality traits, and the presence of anxiety and depression symptoms in a sample of healthy young adults.
A growing body of work from brain imaging research supports the idea that the brain can be conceptualized as a collection of interrelated systems or networks, and that brain regions that are involved in similar processes appear to be interrelated at both structural and functional levels (Alexander-Bloch, Giedd, & Bullmore, Reference Alexander-Bloch, Giedd and Bullmore2013; Bullmore & Sporns, Reference Bullmore and Sporns2009; Dosenbach et al., Reference Dosenbach, Fair, Miezin, Cohen, Wenger, Dosenbach and Petersen2007; Dosenbach et al., Reference Dosenbach, Visscher, Palmer, Miezin, Wenger, Kang and Petersen2006; Mechelli, Friston, Frackowiak, & Price, Reference Mechelli, Friston, Frackowiak and Price2005; Power et al., Reference Power, Cohen, Nelson, Wig, Barnes, Church and Petersen2011; Power & Petersen, Reference Power and Petersen2013; Yeo, Krienen, Chee, & Buckner, Reference Yeo, Krienen, Chee and Buckner2014; Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Buckner2011). Although the exact delineation and dynamics of such networks continues to be an area of debate (e.g., Dosenbach et al., Reference Dosenbach, Visscher, Palmer, Miezin, Wenger, Kang and Petersen2006; Power et al., Reference Power, Cohen, Nelson, Wig, Barnes, Church and Petersen2011; Power & Petersen, Reference Power and Petersen2013; Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Buckner2011), multiple networks appear to play key roles in top-down processing, cognitive control, and integration of emotional information (Dosenbach et al., Reference Dosenbach, Visscher, Palmer, Miezin, Wenger, Kang and Petersen2006; Power et al., Reference Power, Cohen, Nelson, Wig, Barnes, Church and Petersen2011; Power & Petersen, Reference Power and Petersen2013; Seeley et al., Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna and Greicius2007; Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead and Buckner2011). A shared feature of these networks is that key nodes exist within the prefrontal cortex (PFC), which has long been identified as a sector of the brain important for cognitive control and executive function (Gilbert & Burgess, Reference Gilbert and Burgess2008; Miller, Reference Miller2000; Ochsner & Gross, Reference Ochsner and Gross2005). Although the relative unity and diversity of cognitive control functions, and of the PFC, continues to be an ongoing area of research (Collette et al., Reference Collette, Van der Linden, Laureys, Delfiore, Degueldre, Luxen and Salmon2005; Duncan, Johnson, Swales, & Freer, Reference Duncan, Johnson, Swales and Freer1997; Friedman & Miyake, Reference Friedman and Miyake2017; Miller, Reference Miller2000; Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000; Teuber, Reference Teuber1972), available evidence converges on the shared role of PFC regions in functions that contribute to the ability to cope with emotional challenges, such as cognitive reappraisal (Buhle et al., Reference Buhle, Silvers, Wager, Lopez, Onyemekwu, Kober and Ochsner2013; Goldin, McRae, Ramel, & Gross, Reference Goldin, McRae, Ramel and Gross2008), positive affect (Ashby, Isen, & Turken, Reference Ashby, Isen and Turken1999; Davidson & Irwin, Reference Davidson and Irwin1999), and optimism (Dolcos, Hu, Iordan, Moore, & Dolcos, Reference Dolcos, Hu, Iordan, Moore and Dolcos2016; Kringelbach, Reference Kringelbach2005; Sharot, Riccardi, Raio, & Phelps, Reference Sharot, Riccardi, Raio and Phelps2007). We therefore introduce these associations below.
Within the PFC, the middle frontal cortex (MFC), inferior frontal cortex (IFC), and orbital frontal cortex (OFC) have each been linked to integration and control of emotion. Greater engagement of these regions has been found consistently in association with cognitive reappraisal (Buhle et al., Reference Buhle, Silvers, Wager, Lopez, Onyemekwu, Kober and Ochsner2013; Goldin et al., Reference Goldin, McRae, Ramel and Gross2008; Kalisch, Reference Kalisch2009), an emotion regulation strategy that involves construing a particular situation in a way that changes its emotional impact (Gross & John, Reference Gross and John2003; Lazarus & Alfert, Reference Lazarus and Alfert1964). Individual differences in habitual engagement of reappraisal have also been associated with changes in brain response to emotional stimuli in the PFC (Drabant, McRae, Manuck, Hariri, & Gross, Reference Drabant, McRae, Manuck, Hariri and Gross2009). This suggests that the ways in which individuals typically control their emotions impacts neural processing and might, over the course of development, alter the structure of the underlying brain regions. Interestingly, engagement of reappraisal for longer durations seems to shift activity from left to right lateral PFC (Kalisch, Reference Kalisch2009), and habitual engagement of reappraisal is positively associated with the volume of the right MFC (Moore et al., Reference Moore, Iordan, Hu, Kragel, Dolcos and Dolcos2016). The right MFC has also been shown to be negatively associated with symptoms of emotional distress, such as depression (Bora, Fornito, Pantelis, & Yucel, Reference Bora, Fornito, Pantelis and Yucel2012; Chang et al., Reference Chang, Yu, McQuoid, Messer, Taylor, Singh and Payne2011). Together, these results suggest that within the MFC, the volume of the right hemisphere is particularly associated with individual differences in cognitive control of emotion and protection against symptoms of emotional distress.
The PFC has also been identified as playing an important role supporting positive affect (Davidson & Irwin, Reference Davidson and Irwin1999). In particular, convergent evidence from lesions, electroencephalography, and neuroimaging studies suggests that the left PFC is part of a system facilitating approach toward positive affective stimuli (Canli, Desmond, Zhao, Glover, & Gabrieli, Reference Canli, Desmond, Zhao, Glover and Gabrieli1998; Davidson & Irwin, Reference Davidson and Irwin1999; Dolcos, LaBar, & Cabeza, Reference Dolcos, LaBar and Cabeza2004; Eddington, Dolcos, Cabeza, Krishnan, & Strauman, Reference Eddington, Dolcos, Cabeza, Krishnan and Strauman2007; Harmon-Jones, Reference Harmon-Jones2003). The left PFC has also been linked to trait optimism (Dolcos et al., Reference Dolcos, Hu, Iordan, Moore and Dolcos2016), which refers to the dispositional tendency for people to hold generalized favorable expectancies about their future (Carver, Scheier, & Segerstrom, Reference Carver, Scheier and Segerstrom2010). For example, disruption of the left PFC using transcranial magnetic stimulation has been shown to enhance the ability to incorporate negative information into beliefs (Sharot et al., Reference Sharot, Kanai, Marston, Korn, Rees and Dolan2012), suggesting that the left PFC facilitates the optimistic bias. Consistent with this idea, the left IFC and OFC have been shown to be negatively associated with symptoms of anxiety (Hu & Dolcos, Reference Hu and Dolcos2017; Shang et al., Reference Shang, Fu, Ren, Zhang, Du, Gong and … Zhang2014; Talati et al., Reference Talati, Pantazatos, Schneier, Weissman and Hirsch2013) and depression (Bremner et al., Reference Bremner, Vythilingam, Vermetten, Nazeer, Adil, Khan and Charney2002; Lai, Payne, Byrum, Steffens, & Krishnan, Reference Lai, Payne, Byrum, Steffens and Krishnan2000; Shah, Ebmeier, Glabus, & Goodwin, Reference Shah, Ebmeier, Glabus and Goodwin1998). This suggests that within the IFC and OFC, the volume of the left hemisphere is particularly important in supporting cognitive control of emotion and protection against symptoms of emotional distress.
Consistent with the idea that the dispositional traits of cognitive reappraisal, positive affectivity, and optimism protect against symptoms of distress, these factors have been shown to be positively associated with one another (Chang, Maydeu-Olivares, & D’Zurilla, Reference Chang, Maydeu-Olivares and D’Zurilla1997; Gross & John, Reference Gross and John2003), and to negatively predict anxiety and depression (Gross & John, Reference Gross and John2003; Martin & Dahlen, Reference Martin and Dahlen2005; Scheier, Carver, & Bridges, Reference Scheier, Carver and Bridges1994; Watson, Clark, & Tellegen, Reference Watson, Clark and Tellegen1988), suggesting that each of these traits might be an indicator of a common factor indexing well-being. There is also evidence suggesting that each of these constructs is related to cognitive control. For example, reappraisal has been shown to be linked to cognitive control abilities, such as working memory capacity (McRae, Jacobs, Ray, John, & Gross, Reference McRae, Jacobs, Ray, John and Gross2012), positive affect has been shown to be linked to increased cognitive flexibility and reduced perseveration (Dreisbach & Goschke, Reference Dreisbach and Goschke2004), and optimism has been shown to be associated with self-report indices of executive function such as organization (Kruger, Reference Kruger2011). Together, the available evidence suggests that cognitive reappraisal, positive affectivity, and optimism share a common association involving adaptive control of emotion that protects against negative emotional outcomes and emotional distress.
To summarize, greater engagement within a system of PFC regions has been associated with more adaptive responses to emotional challenges (Buhle et al., Reference Buhle, Silvers, Wager, Lopez, Onyemekwu, Kober and Ochsner2013; Davidson & Irwin, Reference Davidson and Irwin1999; Goldin et al., Reference Goldin, McRae, Ramel and Gross2008; Harmon-Jones, Reference Harmon-Jones2003; Kalisch, Reference Kalisch2009), suggesting that a similar pattern may exist at the level of brain structure (Davidson & Irwin, Reference Davidson and Irwin1999; Dolcos et al., Reference Dolcos, Hu, Iordan, Moore and Dolcos2016; Hu & Dolcos, Reference Hu and Dolcos2017; Moore et al., Reference Moore, Iordan, Hu, Kragel, Dolcos and Dolcos2016). The dispositional traits of cognitive reappraisal, positive affectivity, and optimism are personality dimensions that help to protect against symptoms of emotional distress (Carver, Scheier, & Segerstrom, Reference Carver, Scheier and Segerstrom2010; Gross & John, Reference Gross and John2003; Martin & Dahlen, Reference Martin and Dahlen2005; Scheier, Carver, & Bridges, Reference Scheier, Carver and Bridges1994; Watson, Clark, & Tellegen, Reference Watson, Clark and Tellegen1988). Finally, indices of emotional distress, including anxiety and depression, have been linked to reduced volume in PFC structures (Bora et al., Reference Bora, Fornito, Pantelis and Yucel2012; Bremner et al., Reference Bremner, Vythilingam, Vermetten, Nazeer, Adil, Khan and Charney2002; Chang et al., Reference Chang, Yu, McQuoid, Messer, Taylor, Singh and Payne2011; Hu & Dolcos, Reference Hu and Dolcos2017; Lai et al., Reference Lai, Payne, Byrum, Steffens and Krishnan2000; Shah et al., Reference Shah, Ebmeier, Glabus and Goodwin1998; Shang et al., Reference Shang, Fu, Ren, Zhang, Du, Gong and … Zhang2014; Talati et al., Reference Talati, Pantazatos, Schneier, Weissman and Hirsch2013), and reduced indices of the resilience-related personality traits (Carver, Scheier, & Segerstrom, Reference Carver, Scheier and Segerstrom2010; Gross & John, Reference Gross and John2003; Martin & Dahlen, Reference Martin and Dahlen2005; Scheier, Carver, & Bridges, Reference Scheier, Carver and Bridges1994; Watson, Clark, & Tellegen, Reference Watson, Clark and Tellegen1988).
However, what remains unclear is whether the suggested pattern of common factors in brain structure and in personality predicts lower symptoms of distress. To clarify this issue, the current study employed structural equation modeling using a brain-personality-distress symptom framework, and explored a possible mediating role of resilience-related personality traits in the link between PFC volume and measures of distress (i.e., anxiety and depression), in a sample of healthy young adults. The overall concept for the present report was informed by the existing literature (Colibazzi et al., Reference Colibazzi, Zhu, Bansal, Schultz, Wang and Peterson2008; Kim, Zhu, Chang, Bentler, & Ernst, Reference Kim, Zhu, Chang, Bentler and Ernst2007; Marsh et al., Reference Marsh, Ludtke, Muthen, Asparouhov, Morin, Trautwein and Nagengast2010; Yeh et al., Reference Yeh, Zhu, Nicoletti, Hatch, Brambilla and Soares2010), and builds on previous findings coming from our work that targeted specific brain regions and factors (Dolcos et al., Reference Dolcos, Hu, Iordan, Moore and Dolcos2016; Hu & Dolcos, Reference Hu and Dolcos2017; Moore et al., Reference Moore, Iordan, Hu, Kragel, Dolcos and Dolcos2016) with a goal of testing for an integrated and comprehensive model. By incorporating factors that reflect individual differences in PFC volume, personality traits associated with enhanced positive affectivity, and measures of distress, the current study integrates control- and resilience-related constructs in a comprehensive brain-personality-symptom framework. Such an approach has the potential to advance our understanding of resilience and vulnerability to emotional distress and its mechanisms. The current study tested the following hypotheses: (a) A latent construct of PFC volume, including the MFC, IFC, and OFC, would be positively associated with latent trait Resilience; (b) a latent construct of trait Resilience would be negatively associated with Anxiety and Depression; and (c) the latent PFC volume would negatively predict symptoms of Distress through greater latent trait Resilience.
1. Methods
1.1. Participants
Data were collected from a sample of 85 healthy young participants (18–34 years old, 48 females), who had undergone magnetic resonance imaging (MRI) scanning. Some individual differences measures were not completed by all participants (see Analytic Overview subsection and Supplementary Materials Table 1 for details of final sample sizes, as well as details of statistical outlier assessment and removal that preceded all reported results). No participants had previously been diagnosed with any neurological, psychiatric, or personality disorders. Potential outlier cases were assessed and excluded from final analyses, based on procedures described below. The neuropsychological testing and structural brain imaging procedures were part of a common protocol across multiple individual functional brain imaging studies that also involved completion of behavioral tasks in the scanner. Participants completed questionnaires in one or more sessions at a computer terminal in the lab, which typically occurred within a few weeks around the MRI scanning session, depending on the specific functional studies. The present sample overlaps with samples previously reported elsewhere (Dolcos et al., Reference Dolcos, Hu, Iordan, Moore and Dolcos2016; Hu & Dolcos, Reference Hu and Dolcos2017; Moore et al., Reference Moore, Iordan, Hu, Kragel, Dolcos and Dolcos2016). The experimental protocol was approved for ethical treatment of human participants by the institutional Health Research Ethics Board, and participants provided written consent and were compensated with either course credit or money. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
1.2. Structural MRI data acquisition and preprocessing
Anatomical images (3D MPRAGE, repetition time=1,600 ms; echo time=3.82 ms; field of view=256×256 mm2; volume size=112 slices; voxel size=1×1×1 mm3) were obtained using a 1.5 Tesla Siemens Sonata scanner. To examine volumetric associations, brain imaging data were processed using a surface-based morphometric procedure. Surface-based cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis suite (FreeSurfer Version 5.3) (Fischl, Reference Fischl2012), which is freely available to download online (https://surfer.nmr.mgh.harvard.edu/). Specifically, raw DICOM images were imported directly into FreeSurfer (Fischl, Reference Fischl2012), where a semiautomatic workflow was adopted to ensure quality control at the stages of Talairach registration, skull stripping, white matter surface reconstruction, and pial surface reconstruction. Output from each of these stages was visually examined for quality assurance, and major errors were corrected using standard adjustment parameters or manual intervention before rerunning the necessary processing steps again until results were of good quality.
Volume measures from regions of interest (ROIs) were extracted using the parcellation from Desikan et al. (Reference Desikan, Segonne, Fischl, Quinn, Dickerson, Blacker and Killiany2006). Specifically, the right MFC (combined caudal and rostral MFC) ROI identified the region bordered by the superior frontal sulcus, the inferior frontal sulcus, and the precentral sulcus. For the left IFC (pars opercularis) ROI, the whole IFC was identified as the area delineated anteriorly by the rostral extent of the inferior frontal sulcus, posteriorly by the precentral gyrus, laterally by the lateral bank of the inferior frontal sulcus, and medially by the medial bank of the lateral orbital sulcus and/or the circular insular sulcus. The subdivision pars opercularis was defined on this IFC ROI as the first gyrus from the precentral gyrus. Finally, the left OFC (lateral OFC) ROI identified the region lateral to the medial orbital sulcus, within the rostral and caudal extent of the lateral orbital gyrus, bordering the lateral bank of the lateral orbital sulcus and/or the circular insular sulcus at the lateral aspect. Figure 1 shows an example of the ROI delineations. To account for overall brain size differences, the brain region volumes were scaled. Specifically, the MFC, IFC, and OFC volumes were divided by total intracranial volume, then multiplied by a constant (i.e., 1,000), to bring the scaled values to a variance range similar to the other variables in the structural equation model.
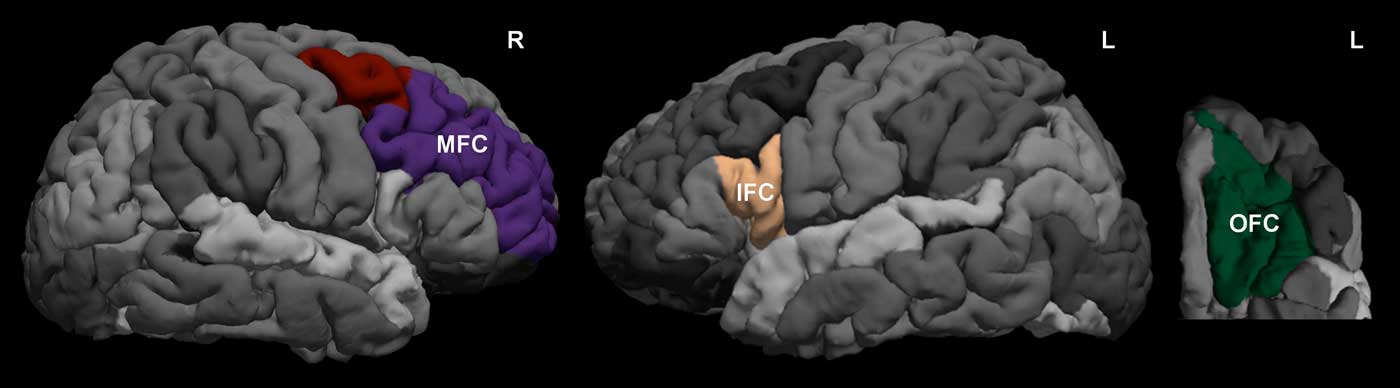
Figure 1 The regions of interest selected for prefrontal cortex volumes. The Desikan-Killiany atlas was used to extract volumes for the MFC, IFC, and OFC for each participant. L = Left; R = Right; MFC = middle frontal cortex; IFC = inferior frontal cortex; OFC = orbital frontal cortex.
1.3. Individual differences measures
Personality and symptom measures included the Emotion Regulation Questionnaire (ERQ) (Gross & John, Reference Gross and John2003), Positive and Negative Affect Schedule (PANAS) (Watson, Clark, & Tellegen, Reference Watson, Clark and Tellegen1988), Life Orientation Test-Revised (LOT-R) (Scheier, Carver, & Bridges, Reference Scheier, Carver and Bridges1994), State-Trait Anxiety Inventory (STAI) (Spielberger, Gorsuch, & Lushene, Reference Spielberger, Gorsuch and Lushene1970), and the Beck Depression Inventory (BDI) (Beck, Ward, Mendelson, Mock, & Erbaugh, Reference Beck, Ward, Mendelson, Mock and Erbaugh1961; Sanz & Garcia-Vera, Reference Sanz and Garcia-Vera2007).
The ERQ assesses the habitual engagement of two emotion regulation strategies, reappraisal and suppression, using a 7-point Likert scale that ranges from 1=“strongly disagree,” to 7=“strongly agree.” Examples of statements from the reappraisal dimension include “I control my emotions by changing the way I think about the situation I’m in,” and statements from the suppression dimension include “I keep my emotions to myself” (Gross & John, Reference Gross and John2003). In this sample, the Cronbach’s α was .73 for reappraisal, and .79 for suppression (n=80).
The PANAS is a widely used measure of current/trait affect (Watson, Clark, & Tellegen, Reference Watson, Clark and Tellegen1988). It includes a list of 20 adjective descriptors of 10 positive (e.g., “interested,” “enthusiastic”) and 10 negative (e.g., “irritable,” “upset”) affects. Items are rated on a 5-point scale from 1=“very slightly or not at all,” to 5=“extremely” according “to what extent [the person] feels this way right now” or during a longer period of time (i.e., “in general”). In the current study, the trait measure for positive affect (Cronbach’s α=.92, n=78) over a longer period of time was used (negative affect Cronbach’s α=.79, n=78).
The LOT-R consists of 10 statements (e.g., “I’m always optimistic about my future,” “I rarely count on good things happening to me”), which measure the degree of optimism or pessimism (Scheier, Carver, & Bridges, Reference Scheier, Carver and Bridges1994). Each statement is rated on a 5-point scale from 0=“strongly disagree,” to 4=“strongly agree.” Cronbach’s α was .76 in this sample (n=58).
The STAI provides measures of the temporary condition of “state anxiety” and the more general and long-standing quality of “trait anxiety” in adults (Spielberger, Gorsuch, & Lushene, Reference Spielberger, Gorsuch and Lushene1970). The STAI consists of two scales containing 20 items each, with the trait anxiety measure evaluating how the participant feels “generally.” It uses “I feel/I am” statements that are rated on 4-point scale from 1=“not at all,” to 4=“very much so.” In the current study, the total trait measure of how a participant feels generally was used (Cronbach’s α=.88, n=81).
Finally, the BDI is a commonly used measure of depression (Beck et al., Reference Beck, Ward, Mendelson, Mock and Erbaugh1961; Sanz & Garcia-Vera, Reference Sanz and Garcia-Vera2007). It consists of 21 items, each of them having four possible options to select from, ranging in intensity from 0 to 3 (e.g., 0=“I do not feel sad;” 1=“I feel sad;” 2=“I am sad all the time and I can’t snap out of it;” 3=“I am so sad or unhappy that I can’t stand it”). A value of 0–3 is assigned to each item and the total score determines the depression severity, the higher the score the more severe the depression (Cronbach’s α=.80, n=79).
1.4. Analytic overview
Structural MRI data were analyzed in conjunction with the individual difference measures introduced above, to examine associations among brain structure, personality, and distress symptoms. Analyses were carried out for testing statistical models involving brain region volumes, personality measures, and symptom measures, using R 3.4.3 with RStudio 1.1.423 and statistical package lavaan (Rosseel, Reference Rosseel2012). The models that were tested were informed by the available anatomical literature, as well as theory regarding factors of resilience and emotional distress. Data were first assessed for potential outlier cases at a univariate level using a criterion of 3 SDs (Osborne & Overbay, Reference Osborne and Overbay2004), for brain, trait, and symptom measures. Four participants were excluded from final analyses. Two of the participants were excluded because of outlier scaled ROI volumes, one participant was excluded because of outlier scores on reappraisal, anxiety, and depression measures, and one participant was excluded because of outlier score on optimism. In addition, some trait and symptom measures were not completed by all participants, thus handling of missing data is described below and final sample sizes are noted in the Supplementary Materials. Supplementary Materials Figure 1 shows scatterplots of the questionnaire data after outlier removal.
Path analyses were conducted using R package lavaan (Rosseel, Reference Rosseel2012). Analyses completed in lavaan used settings that parallel other software packages such as AMOS standard settings, including Wishart estimation, maximum likelihood estimation for handling missing data, and the use of expected information for estimating standard error variance (Arbuckle, Reference Arbuckle2016; Rosseel, Reference Rosseel2012). Within the hypothetical model, a latent factor was constructed for volumes of a PFC brain system of Control, another latent factor was constructed for a Resilience personality variable, and Distress was represented as manifest variables for total Anxiety and total Depression. Variables of sex and age were included in the regressions on the mediator and symptom variables, to control for the influence of these demographics. Given that a reversal of the proposed direction of effects is also statistically plausible, we tested two alternative models to examine the possibility that Resilience mediates the link from Distress to Control, or that Control mediates the link from Resilience to Distress. To determine the model fit, we examined the χ2/df ratio, comparative fit index (CFI), and root mean square error of approximation (RMSEA). A good model fit is reflected by χ2/df ratios <3 (Kline, Reference Kline1998), fit indices above .90 (Bentler, Reference Bentler1990; Kline, Reference Kline1998), and RMSEA values ≤.08.
2. Results
Analyses were conducted on brain, personality, and distress measures using the hypothetical structural equation model. The structural equation model included confirmatory factor analysis of the manifest brain and personality variables into latent variable constructs, which then were tested for predicted associations among each other and anxiety and depression measures using regression and mediation analyses. Supplementary Materials Table 1 provides descriptive information and intercorrelations for the targeted variables.
Figure 2 displays the latent variable mediation model for statistically predicting Anxiety with standardized path coefficients. The model predicting Anxiety showed a strong fit to the data, χ2(22)=21.16, ns, χ2/df=.96, CFI=1.00, RMSEA=.00. As expected, the scaled PFC volumes contributed significantly to the latent construct of Control (ps<.001), and the personality traits of reappraisal, positive affectivity, and optimism contributed significantly to the latent construct of Resilience (ps<.01). Consistent with the idea that brain regions engaged in cognitive control are associated with protection against emotional distress, the latent construct of PFC system volume positively predicted the latent construct of trait Resilience, and latent trait Resilience negatively predicted Anxiety. Furthermore, mediation analysis confirmed that greater latent PFC volume is indirectly associated with lower Anxiety symptoms through greater latent trait Resilience (see Figure 2).
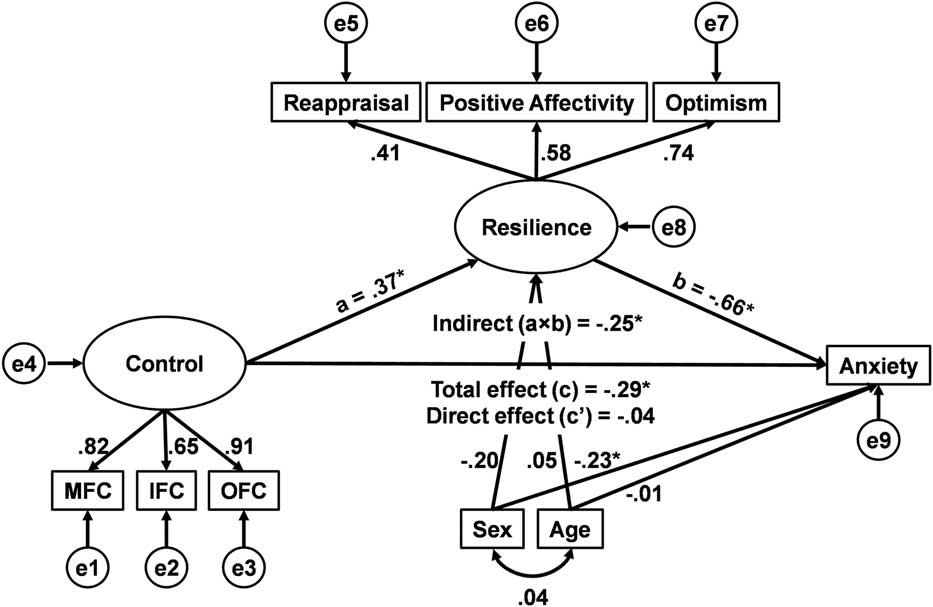
Figure 2 Structural equation model of latent prefrontal cortical (PFC) volume, latent trait Resilience, and Anxiety. Results from the proposed model confirm that latent PFC volume is associated with lower Anxiety, through greater latent trait Resilience. Standardized coefficients are shown for each path. MFC=middle frontal cortex (right side); IFC=inferior frontal cortex (left side); OFC=orbital frontal cortex (left side); e1–9=error terms. *Indicates p<.05 for the mediation analysis.
Consistent with the idea that anxiety and depression are often comorbid but also have nonoverlapping aspects (Bishop & Forster, Reference Bishop and Forster2013; Fava et al., Reference Fava, Rankin, Wright, Alpert, Nierenberg, Pava and Rosenbaum2000; Ormel et al., Reference Ormel, Bastiaansen, Riese, Bos, Servaas, Ellenbogen and Aleman2013; Pollack, Reference Pollack2005), the hypothesized model did not work as well for statistically predicting Depression. Specifically, fitting the same model that was used for predicting Anxiety to predict Depression showed an overall good fit to the data, χ2(22)=21.32, ns, χ2/df=.97, CFI=1.00, RMSEA=.00, but mediation analysis revealed only a trend level of significance for an indirect effect (a=.32, p=.074; b=−.56, p=.018; c=−.12, p=.299; c′=.06, p=.626; ab=−.18, p=.058). This possibly suggests that a similar model could be helpful for examining Depression, but that the current model might be better suited for understanding and predicting Anxiety in particular.
As expected, although the alternative model testing whether Resilience mediates the link from Anxiety to Control provided a good fit to the data, χ2(20)=21.06, ns, χ2/df=1.05, CFI=.99, RMSEA=.03, paths from Anxiety and Resilience to Control were not significant and a significant indirect effect was not found (ps>.19). The alternative model testing whether Control mediates the link from Resilience to Anxiety also provided a good fit, χ2(22)=23.06, ns, χ2/df=1.05, CFI=.99, RMSEA=.02, but the path from Control to Anxiety was not significant, and a significant indirect effect was also not found (ps>.68). For Depression, the alternative model testing whether Resilience mediates the link from Depression to Control provided a good fit, χ2(20)=21.25, ns, χ2/df=1.06, CFI=.99, RMSEA=.03, but paths from Depression and Resilience to Control were not significant and a significant indirect effect was not found (ps>.08). The alternative model testing whether Control mediates the link from Resilience to Depression also provided a good fit, χ2(22)=25.61, ns, χ2/df=1.16, CFI=.97, RMSEA=.05, but the path from Control to Depression was not significant, and a significant indirect effect was not found (ps>.56). In addition, results from supplementary analyses supported the idea that the associations identified among the targeted brain regions, personality traits, and symptoms are specific. Tests of alternative or additional brain, personality, and symptom measures did not appear to work better than the featured model (see Supplementary Materials).
3. Discussion
The present study demonstrated the successful implementation of a structural equation modeling approach to a brain-personality-distress symptom framework. Results showed that within an integrative structural equation model, latent factors of PFC brain volume and trait Resilience could be constructed and examined in association with symptoms of Anxiety and Depression. As expected, results showed that the latent construct of PFC volumes positively predicted the latent construct of Resilience, which in turn negatively predicted Anxiety. Furthermore, mediation analysis confirmed that greater latent PFC volume is indirectly associated with lower Anxiety symptoms through greater latent trait Resilience. Interestingly, the model fit well for Anxiety but did not show a significant mediation for Depression, which tentatively suggests that the associations with Anxiety are clearer, and more research will be needed to clarify the associations with Depression.
The latent construct of PFC region volumes is consistent with recent work suggesting an underlying framework of structural covariance in the brain (Alexander-Bloch, Giedd, & Bullmore, Reference Alexander-Bloch, Giedd and Bullmore2013; Baskin-Sommers, Neumann, Cope, & Kiehl, Reference Baskin-Sommers, Neumann, Cope and Kiehl2016; Bullmore & Sporns, Reference Bullmore and Sporns2009; Colibazzi et al., Reference Colibazzi, Zhu, Bansal, Schultz, Wang and Peterson2008; Mechelli et al., Reference Mechelli, Friston, Frackowiak and Price2005; Yeh et al., Reference Yeh, Zhu, Nicoletti, Hatch, Brambilla and Soares2010), and extends this idea to a system of PFC regions associated with the integration and control of emotion. The volumetric association between regions of the brain that are functionally related is consistent with the idea of use-dependent plasticity (Bütefisch et al., Reference Bütefisch, Davis, Wise, Sawaki, Kopylev, Classen and Cohen2000; Nudo, Milliken, Jenkins, & Merzenich, Reference Nudo, Milliken, Jenkins and Merzenich1996; for reviews of relevant studies in humans, see Draganski & May, Reference Draganski and May2008; May, Reference May2011), which suggests that repeated patterns of neuronal firing may lead to increased synaptic connectivity (Hebb, Reference Hebb1949) and increases in gray matter volume (Draganski et al., Reference Draganski, Gaser, Kempermann, Kuhn, Winkler, Buchel and May2006). Furthermore, the present results suggest that latent variable analysis is a feasible way of assessing the structural associations of functionally related brain regions, which complements other structural and functional approaches commonly used in the field to assess brain systems and networks (Bullmore & Sporns, Reference Bullmore and Sporns2009).
The contribution of the right MFC to the latent factor of Control is consistent with evidence that the right PFC is associated with engagement of reappraisal to decrease negative emotional response (Ochsner, Silvers, & Buhle, Reference Ochsner, Silvers and Buhle2012), and with a system facilitating avoidance of aversive stimuli (Canli et al., Reference Canli, Desmond, Zhao, Glover and Gabrieli1998; Davidson & Irwin, Reference Davidson and Irwin1999; Dolcos, LaBar & Cabeza, Reference Dolcos, LaBar and Cabeza2004; Eddington et al., Reference Eddington, Dolcos, Cabeza, Krishnan and Strauman2007; Spielberg, Stewart, Levin, Miller, & Heller, Reference Spielberg, Stewart, Levin, Miller and Heller2008). In addition, the MFC has been emphasized in the integration of emotion and cognition (Gray, Braver, & Raichle, Reference Gray, Braver and Raichle2002), and in executive processes such as working memory (Curtis & D’Esposito, Reference Curtis and D’Esposito2003), suggesting that there are multiple possibly interrelated processes that engage the MFC. Consistent with this idea, in the present study, MFC volume tended to be positively associated with Resilience-related traits and negatively associated with Anxiety. Taken together, these findings suggest that the MFC plays a key role in the integration and control of emotion, and that this role might involve or emerge from common functions that engage this brain region along with the IFC and OFC.
The left IFC and OFC contribution to the latent PFC construct is consistent with evidence that the left PFC is involved in a system facilitating approach toward appetitive stimuli (Davidson & Irwin, Reference Davidson and Irwin1999), protecting against anxiety (Hu & Dolcos, Reference Hu and Dolcos2017; Shang et al., Reference Shang, Fu, Ren, Zhang, Du, Gong and … Zhang2014; Talati et al., Reference Talati, Pantazatos, Schneier, Weissman and Hirsch2013), and supporting optimism (Dolcos et al., Reference Dolcos, Hu, Iordan, Moore and Dolcos2016). The present findings suggest that larger volume of left PFC protects against symptoms of Anxiety, and that the OFC and MFC share positive associations with optimism (see Supplementary Materials Table 1). This is in line with the notion that optimism is a higher level trait that, beyond reflecting reward-related processing, also reflects individual differences in self-regulation and goal-directed behavior (Carver, Scheier, & Segerstrom, Reference Carver, Scheier and Segerstrom2010; Nes & Segerstrom, Reference Nes and Segerstrom2006).
The latent construct of trait Resilience is consistent with the idea that cognitive reappraisal, positive affectivity, and optimism are associated traits (Chang, Maydeu-Olivares, & D’Zurilla, Reference Chang, Maydeu-Olivares and D’Zurilla1997; Gross & John, Reference Gross and John2003), that each tap into a common factor that protects against emotional challenges. These traits also appear to be associated with individual differences in cognitive control and executive functions (Dreisbach & Goschke, Reference Dreisbach and Goschke2004; Kruger, Reference Kruger2011; McRae et al., Reference McRae, Jacobs, Ray, John and Gross2012), which supports the idea of examining latent constructs that might pull out common variance from across the observed personality traits. For example, it is interesting that positive affectivity was not directly correlated with any of the PFC volumes. However, positive affectivity contributed to the latent Resilience trait that was associated with PFC system volume, which suggests a more complex relation between a very general emotional trait, such as positive affectivity, and PFC volume. It is possible that general emotional traits such as positive affectivity have a diffuse association with brain volume, which is more readily captured with latent construct analyses as opposed to manifest variable assessments.
The strong fit of the structural equation model and significant prediction of Anxiety is consistent with previous evidence showing negative associations between PFC volume and anxiety (Dolcos et al., Reference Dolcos, Hu, Iordan, Moore and Dolcos2016; Hu & Dolcos, Reference Hu and Dolcos2017; Shang et al., Reference Shang, Fu, Ren, Zhang, Du, Gong and … Zhang2014; Talati et al., Reference Talati, Pantazatos, Schneier, Weissman and Hirsch2013). It is also consistent with previous evidence showing that cognitive reappraisal, positive affect, and optimism negatively predict anxiety (Gross & John, Reference Gross and John2003; Martin & Dahlen, Reference Martin and Dahlen2005). Together with the significant mediation, these findings suggest that, while there are common factors at the levels of PFC system volume and trait Resilience, the current model primarily describes interrelations that are associated with Anxiety. Interestingly, the model did not work as well for Depression, which was also not directly correlated with any of the scaled PFC volumes in this sample. This possibly suggests that, although the regions included here have been shown to be associated with depression in other research (Bora et al., Reference Bora, Fornito, Pantelis and Yucel2012; Bremner et al., Reference Bremner, Vythilingam, Vermetten, Nazeer, Adil, Khan and Charney2002; Chang et al., Reference Chang, Yu, McQuoid, Messer, Taylor, Singh and Payne2011; Lai et al., Reference Lai, Payne, Byrum, Steffens and Krishnan2000; Shah et al., Reference Shah, Ebmeier, Glabus and Goodwin1998), perhaps other regions that appear to be affected by depression would fit better in a latent factor analysis targeting depression specifically.
It is also possible that the results were somewhat influenced by aspects of the anxiety and depression measures themselves. The present study used a specific version of the STAI measure that targets trait anxiety, whereas the BDI typically assesses symptoms in a time range that includes the day of assessment. To further clarify these aspects, it would be important to investigate other brain regions that are also implicated in emotional dysregulation (Mayberg, Reference Mayberg1997, Reference Mayberg2006), and perhaps further explore other measures that are associated with emotional distress (e.g., neuroticism, Costa & McCrae, Reference Costa and McCrae1992). At any rate, the present mediation results are more interpretable in the prediction of anxiety symptoms, and future research is needed with regard to depression.
Overall, the present mediation findings are important because they suggest that, by modifying brain- and/or personality-level factors, it might be possible to change behavioral-level outcomes reflected in symptoms of anxiety, even within the spectrum of healthy functioning. The volume of PFC regions has been shown to change in response to experience and training (May, Reference May2011), and interventions designed to train cognitive control of emotion hold promise in alleviating symptoms of emotional distress and affective disturbances (Fava et al., Reference Fava, Rankin, Wright, Alpert, Nierenberg, Pava and Rosenbaum2000; Siegle, Ghinassi, & Thase, Reference Siegle, Ghinassi and Thase2007). The plasticity of brain structures and trait-level resilience factors reflects the dynamic interaction between the brain and behavior, and points to the possibility that resilience and well-being can be enhanced through training (Davidson & McEwen, Reference Davidson and McEwen2012). Hence, by identifying concrete brain and personality factors that protect against symptoms of emotional distress, the present investigation highlights possible targets and related training areas (e.g., executive function, emotion control) for future interventions. To further investigate this possibility, future research could target tasks related to cognitive/executive control and emotion processing (e.g., Affective Go/No-Go task; Hu & Dolcos, Reference Hu and Dolcos2017).
Since a common function of the presently identified PFC system appears to be the cognitive control of emotion, future work should test the association of such a PFC system with other indicators of cognitive control, to tease apart the different roles that the system might play compared to other systems or networks. For example, externalizing and substance abuse are important factors often examined in clinical research, which might be linked to these brain regions, but might also be linked to other systems such as fronto-striatal circuits (Limbrick-Oldfield, van Holst, & Clark, Reference Limbrick-Oldfield, van Holst and Clark2013; Shannon, Sauder, Beauchaine, & Gatzke-Kopp, Reference Shannon, Sauder, Beauchaine and Gatzke-Kopp2009). Regions such as the anterior cingulate cortex (ACC) also emerge in the literature related to cognitive control of emotion, resilience, anxiety, and depression, but the commonality of these associations is less clear. More specifically, it has long been posited that within the ACC the dorsal anterior portion might be relatively more involved in “cognitive” processes and the ventral anterior portion might be relatively more involved in “affective” processes (Bush, Luu, & Posner, Reference Bush, Luu and Posner2000). This made the inclusion of the ACC a challenge for the current study, which aimed at making initial steps in identifying regions involved in the integration and control of emotion to test for a common latent factor. On the one hand, the available literature has shown that while the volume of dorsal ACC is associated with habitual reappraisal, the volume of the ventral ACC is not (Giuliani, Drabant, & Gross, Reference Giuliani, Drabant and Gross2011). On the other hand, response in the ventral ACC has been shown to be positively associated with optimism (Sharot et al., Reference Sharot, Riccardi, Raio and Phelps2007). These mixed results suggest that although the ACC plays a key role in the processes targeted in the present study, it might be a heterogeneous and complex role, and hence it should be targeted in future research building upon these initial findings. Other regions such as the amygdala are also important to consider given their interaction with the PFC during cognitive control of emotion (e.g., Buhle et al., Reference Buhle, Silvers, Wager, Lopez, Onyemekwu, Kober and Ochsner2013; Denkova, Dolcos, & Dolcos, Reference Denkova, Dolcos and Dolcos2015; Dolcos, Kragel, Wang, & McCarthy, Reference Dolcos, Kragel, Wang and McCarthy2006; Goldin et al., Reference Goldin, McRae, Ramel and Gross2008), and should be examined in future investigations building on the presented model. We opted to not target regions such as the amygdala in the current analysis, because our previous work has indicated that automated tools such as the one used here for extracting cortical parcellations are not as ideal as manual tracing for extracting region volume in the medial temporal lobe (Hu et al., Reference Hu, Moore, Bertels, Phan, Dolcos and Dolcos2018; Moore et al., Reference Moore, Hu, Woo, O’Hearn, Iordan, Dolcos and Dolcos2014). Overall, the present study provides insights that can guide future research targeting latent constructs of brain structure and function to further elucidate these relations and interactions.
Caveats. First, mediation models of cross-sectional data are limited in the extent to which they can explain dynamic relations among the variables being examined, and thus further empirical studies are needed to verify the directionality of these relations by manipulating and assessing changes at different levels in a longitudinal design. With this caveat in mind, based on the current results, it appears more likely that changes at the brain level, such as trainings that would target PFC-related cognitive control functions, may help to strengthen favorable effects of greater trait Resilience and reduced symptoms of Distress. These results point to promising possible future avenues for intervention studies in healthy populations, and provide novel insights with valuable implications for understanding how these mechanisms might be altered in clinical groups, which should also be tested in future studies. Second, it would also be ideal to have a larger sample size with statistical power that allows for the inclusion of more variables and the use of more conservative statistical criteria, including correction for multiple comparisons, which were not applied here. Third, in the present report, we tested for sex differences at the level of between-group main effects for each variable, and then included sex as a variable of no interest in the structural equation models. This is consistent with common practice in the literature and with previous findings that did not identify sex differences for some of the variables targeted here (Llewellyn, Dolcos, Iordan, Rudolph, & Dolcos, Reference Llewellyn, Dolcos, Iordan, Rudolph and Dolcos2013). However, future research would ideally expand on the current study with larger sample sizes and multigroup analyses to further tease apart possible sex differences with appropriate statistical power.
Fourth, when targeting regions of the brain for analysis, it is important to consider the delineation used to define the ROIs. In the present analysis, a standard anatomical atlas was used to define and extract ROI volumes (see Figure 1). However, there are many possible anatomically or functionally informed atlases that are commonly used (e.g., Destrieux, Fischl, Dale, & Halgren, Reference Destrieux, Fischl, Dale and Halgren2010), and the use of different atlases may contribute to variability in reported findings in the literature. Future work should further examine the brain structural correlates of emotional integration and control using multiple methods. Finally, further testing for specificity (e.g., to anxiety vs. depression) is also important. Consistent with the primary analyses, the results of the main model when tested for Anxiety with univariate statistical outliers included showed the same pattern of associations. Interestingly, when tested for Depression with outliers included, the mediation for Depression appeared to go from marginal to significant. However, we are cautious in interpreting this result as it appears to possibly be driven by particular outlier cases. For example, one participant was a statistical outlier on multiple questionnaire measures, including Depression, which might indicate that this person did not complete the measures accurately, or is potentially outside the spectrum of “typical” healthy individual differences. With this in mind, we have chosen to be on the conservative side and focus on the results without these cases. Nevertheless, as noted above, future research further targeting possible dissociations between anxiety and depression is needed.
4. Conclusion
In summary, the current study showed that greater latent PFC volume in healthy participants was associated with greater latent trait Resilience, which in turn was associated with lower Anxiety. In addition, latent trait Resilience mediated the indirect relation between the latent PFC volume and Anxiety. These results build upon and advance previous findings regarding the roles of the PFC and trait Resilience in the integration and control of emotion to protect against affective challenges. The present findings have valuable implications for the development of future tools targeting the reduction of anxiety, as well as the promotion of enhanced emotional well-being, in both healthy and clinical populations.
Conflicts of Interest:
The authors have nothing to disclose.
Acknowledgments:
This work was conducted in part at the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign (UIUC-BI). The authors wish to thank members of the Dolcos Lab for assisting with data collection. To access analysis scripts, please visit https://github.com/mmoore16/paper-persneuro
Authors’ Contributions: S.D. and F.D. conceived the study; S.D. contributed to data collection; M.M., S.C., S.D., and F.D. contributed to the analytical approach, with feedback from K.L.P. and T.J.S.; M.M. performed the analyses; M.M., S.D., and F.D. wrote the manuscript. All authors provided feedback to, and approved the content of the manuscript.
Financial Support: This research was partially supported by the National Alliance for Research on Schizophrenia and Depression (currently, the Brain & Behavior Research Foundation), the Canadian Psychiatric Research Foundation (currently, Healthy Minds Canada), the Canadian Institutes of Health Research, the University of Alberta Hospital Foundation, and the University of Illinois. During the preparation of this manuscript, F.D. was supported by a Helen Corley Petit Scholarship in Liberal Arts and Sciences and an Emanuel Donchin Professorial Scholarship in Psychology from the University of Illinois. M.M. was supported by a Beckman Institute Pre-Doctoral Fellowship.
Supplementary Material:
To view supplementary material for this article, please visit https://doi.org/10.1017/pen.2018.11






