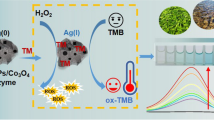
Abstract
When nanozymes are used in biological analysis, higher activity can improve the detection sensitivity, and better selectivity can eliminate other interference. To improve the specificity and sensitivity, we fabricated an innovative bioconjugated nanozyme with natural enzyme (BNNZ), in which natural ChOx was immobilized onto histidine-modified Fe3O4 (His–Fe3O4) with hydrophilic poly(ethylene glycol) (PEG) as a linker. ChOx could specifically catalyze the oxidation of cholesterol to generate H2O2 molecule, and then the newly formed H2O2 oxidized the colorless 3,3′,5,5′-tetramethylbenzidine (TMB) into blue ox-TMB by peroxidase-like His–Fe3O4. According to the above cascade reaction, the BNNZ-based colorimetric strategy was proposed for the detection of cholesterol. Wherein, natural enzymes specifically catalyzed substrates, which endowed BNNZ with excellent specificity for target molecules; meanwhile, the introduction of histidine on His–Fe3O4 effectively increased the peroxidase-like activity of BNNZ, which provided a guarantee for sensitivity. Furthermore, BNNZ after reaction could be rapidly separated by an external magnetic field without interfering with colorimetric quantitative detection. The proposed strategy exhibited excellent sensitivity with limit of detection of 0.446 μM and was successfully used for the detection of cholesterol in spiked human serum sample with recovery and relative standard deviation in the range of 97.9–103.5% and 2.5–4.0%, respectively. This work indicates that the bioconjugation of nanozyme and natural enzyme may be a universal strategy for synthesis of high-performance enzyme–nanozyme systems, and the new-type BNNZ will be widely used in biological detection and disease treatment.
Graphical abstract






Similar content being viewed by others

References
R. Zhang, X. Yan, K. Fan, Nanozymes inspired by natural enzymes. Acc. Mater. Res. 2(7), 534–547 (2021)
Y. Huang, J. Ren, X. Qu, Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 119(6), 4357–4412 (2019)
M. Liang, X. Yan, Nanozymes: from new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 52(8), 2190–2200 (2019)
H. Jin, D. Ye, L. Shen, R. Fu, Y. Tang, J.C.Y. Jung, H. Zhao, J. Zhang, Perspective for single atom nanozymes based sensors: advanced materials, sensing mechanism, selectivity regulation, and applications. Anal. Chem. 94(3), 1499–1509 (2022)
B. Das, J.L. Franco, N. Logan, P. Balasubramanian, M.I. Kim, C. Cao, Nanozymes in point-of-care diagnosis: an emerging futuristic approach for biosensing. Nano-Micro Lett. 13(1), 193 (2021)
M. Wei, J. Lee, F. Xia, P. Lin, X. Hu, F. Li, D. Ling, Chemical design of nanozymes for biomedical applications. Acta Biomater. 126, 15–30 (2021)
L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett, X. Yan, Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2(9), 577–583 (2007)
Y. Hu, H. Cheng, X. Zhao, J. Wu, F. Muhammad, S. Lin, J. He, L. Zhou, C. Zhang, Y. Deng, P. Wang, Z. Zhou, S. Nie, H. Wei, Surface-enhanced Raman scattering active gold nanoparticles with enzyme-mimicking activities for measuring glucose and lactate in living tissues. ACS Nano 11(6), 5558–5566 (2017)
X. Wang, F. Wen, L. He, J. Su, P. Jiang, D. He, Engineering porous Co–Mn oxide nanosheets with abundant oxygen vacancy as an efficient oxidase-like mimic for heparin colorimetric sensing. Anal. Chim. Acta 1198, 339564 (2022)
X. Meng, D. Li, L. Chen, H. He, Q. Wang, C. Hong, J. He, X. Gao, Y. Yang, B. Jiang, G. Nie, X. Yan, L. Gao, K. Fan, High-performance self-cascade pyrite nanozymes for apoptosis–ferroptosis synergistic tumor therapy. ACS Nano 15(3), 5735–5751 (2021)
N. Wang, J. Shi, Y. Liu, W. Sun, X. Su, Constructing bifunctional metal–organic framework based nanozymes with fluorescence and oxidase activity for the dual-channel detection of butyrylcholinesterase. Anal. Chim. Acta 1205, 339717 (2022)
H. Sun, A. Zhao, N. Gao, K. Li, J. Ren, X. Qu, Deciphering a nanocarbon-based artificial peroxidase: chemical identification of the catalytically active and substrate-binding sites on graphene quantum dots. Angew. Chem. Int. Ed. 54(24), 7176–7180 (2015)
T. Zhan, J. Kang, X. Li, L. Pan, G. Li, W. Hou, NiFe layered double hydroxide nanosheets as an efficiently mimic enzyme for colorimetric determination of glucose and H2O2. Sens. Actuators B Chem. 255, 2635–2642 (2018)
R. Cai, D. Yang, S. Peng, X. Chen, Y. Huang, Y. Liu, W. Hou, S. Yang, Z. Liu, W. Tan, Single nanoparticle to 3D supercage: framing for an artificial enzyme system. J. Am. Chem. Soc. 137(43), 13957–13963 (2015)
R. Cai, D. Yang, K. Lin, T.S. Cao, Y. Lyv, K. Chen, Y. Yang, J. Ge, L. Xia, G. Christou, Y. Zhao, Z. Chen, W. Tan, 3D halos assembled from Fe3O4/Au NPs with enhanced catalytic and optical properties. Nanoscale 11(43), 20968–20976 (2019)
H. Wei, E. Wang, Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 80(6), 2250–2254 (2008)
A.A. Vernekar, T. Das, S. Ghosh, G. Mugesh, A remarkably efficient MnFe2O4-based oxidase nanozyme. Chem. Asian J. 11(1), 72–76 (2016)
Z. Chen, J.J. Yin, Y.T. Zhou, Y. Zhang, L. Song, M. Song, S. Hu, N. Gu, Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano 6(5), 4001–4012 (2012)
M.L. Ye, Y. Zhu, Y. Lu, L. Gan, Y. Zhang, Y.G. Zhao, Magnetic nanomaterials with unique nanozymes-like characteristics for colorimetric sensors: a review. Talanta 230, 122299 (2021)
S. Li, Y. Zhang, Q. Wang, A. Lin, H. Wei, Nanozyme-enabled analytical chemistry. Anal. Chem. 94(1), 312–323 (2022)
M.S. Kim, J. Lee, H.S. Kim, A. Cho, K.H. Shim, T.N. Le, S.S.A. An, J.W. Han, M.I. Kim, J. Lee, Heme cofactor-resembling Fe–N single site embedded graphene as nanozymes to selectively detect H2O2 with high sensitivity. Adv. Funct. Mater. 30(1), 1905410 (2020)
W. Wu, Q. Wang, J. Chen, L. Huang, H. Zhang, K. Rong, S. Dong, Biomimetic design for enhancing the peroxidase mimicking activity of hemin. Nanoscale 11(26), 12603–12609 (2019)
K. Fan, H. Wang, J. Xi, Q. Liu, X. Meng, D. Duan, L. Gao, X. Yan, Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem. Commun. 53(2), 424–427 (2017)
L. Dewangan, J. Korram, I. Karbhal, R. Nagwanshi, M.L. Satnami, N-doped carbon quantum dot-MnO2 nanowire FRET pairs: detection of cholesterol, glutathione, acetylcholinesterase, and chlorpyrifos. ACS Appl. Nano Mater. 4(12), 13612–13624 (2021)
C. Hong, L. Chen, C. Wu, D. Yang, J. Dai, Z. Huang, R. Cai, W. Tan, Green synthesis of Au@WSe2 hybrid nanostructures with the enhanced peroxidase-like activity for sensitive colorimetric detection of glucose. Nano Res. 15(2), 1587–1592 (2022)
T.L. Steck, Y. Lange, Cell cholesterol homeostasis: mediation by active cholesterol. Trends Cell Biol. 20(11), 680–687 (2010)
J. Zhang, Q. Liu, Cholesterol metabolism and homeostasis in the brain. Protein Cell 6(4), 254–264 (2015)
G. Llaverias, C. Danilo, I. Mercier, K. Daumer, F. Capozza, T.M. Williams, F. Sotgia, M.P. Lisanti, P.G. Frank, Role of cholesterol in the development and progression of breast cancer. Am. J. Pathol. 178(1), 402–412 (2011)
F.J. Alenghat, A.M. Davis, Management of blood cholesterol. JAMA 321(8), 800–801 (2019)
F.R. Maxfield, I. Tabas, Role of cholesterol and lipid organization in disease. Nature 438(7068), 612–621 (2005)
V. Narwal, R. Deswal, B. Batra, V. Kalra, R. Hooda, M. Sharma, J.S. Rana, Cholesterol biosensors: a review. Steroids 143, 6–17 (2019)
L. Dewangan, J. Korram, I. Karbhal, R. Nagwanshi, V.K. Jena, M.L. Satnami, A colorimetric nanoprobe based on enzyme-immobilized silver nanoparticles for the efficient detection of cholesterol. RSC Adv. 9(72), 42085–42095 (2019)
M. Chung, Y. Jang, M. Kim, Convenient colorimetric detection of cholesterol using multi-enzyme Co-incorporated organic-inorganic hybrid nanoflowers. J. Nanosci. Nanotechnol. 18(9), 6555–6561 (2018)
C. Hong, X. Zhang, C. Wu, Q. Chen, H. Yang, D. Yang, Z. Huang, R. Cai, W. Tan, On-site colorimetric detection of cholesterol based on polypyrrole nanoparticles. ACS Appl. Mater. Interfaces 12(49), 54426–54432 (2020)
Q. Wu, L. He, Z.W. Jiang, Y. Li, Z.M. Cao, C.Z. Huang, Y.F. Li, CuO nanoparticles derived from metal-organic gel with excellent electrocatalytic and peroxidase-mimicking activities for glucose and cholesterol detection. Biosens. Bioelectron. 145, 111704 (2019)
L. Zhao, Z. Wu, G. Liu, H. Lu, Y. Gao, F. Liu, C. Wang, J. Cui, G. Lu, High-activity Mo, S co-doped carbon quantum dot nanozyme-based cascade colorimetric biosensor for sensitive detection of cholesterol. J. Mater. Chem. B 7(44), 7042–7051 (2019)
J. Li, T. Liu, R.A. Dahlgren, H. Ye, Q. Wang, Y. Ding, M. Gao, X. Wang, H. Wang, N, S-co-doped carbon/Co1-xS nanocomposite with dual-enzyme activities for a smartphone-based colorimetric assay of total cholesterol in human serum. Anal. Chim. Acta 1204, 339703 (2022)
G.I. Berglund, G.H. Carlsson, A.T. Smith, H. Szöke, A. Henriksen, J. Hajdu, The catalytic pathway of horseradish peroxidase at high resolution. Nature 417(6887), 463–468 (2002)
Z. Mu, S. Wu, J. Guo, M. Zhao, Y. Wang, Dual mechanism enhanced peroxidase-like activity of iron–nickel bimetal–organic framework nanozyme and its application for biosensing. ACS Sustain. Chem. Eng. 10(9), 2984–2993 (2022)
M.A. Voinov, J.O.S. Pagán, E. Morrison, T.I. Smirnova, A.I. Smirnov, Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. J. Am. Chem. Soc. 133(1), 35–41 (2011)
Q. Liu, A. Zhang, R. Wang, Q. Zhang, D. Cui, A review on metal- and metal oxide-based nanozymes: properties, mechanisms, and applications. Nano-Micro Lett. 13(1), 154 (2021)
C. Zhang, C. Chen, D. Zhao, G. Kang, F. Liu, F. Yang, Y. Lu, J. Sun, Multienzyme cascades based on highly efficient metal–nitrogen–carbon nanozymes for construction of versatile bioassays. Anal. Chem. 94(8), 3485–3493 (2022)
A. Menotti, M. Lanti, A. Zanchetti, G. Botta, M. Laurenzi, O. Terradura-Vagnarelli, M. Mancini, The role of HDL cholesterol in metabolic syndrome predicting cardiovascular events. The Gubbio population study. Nutr. Metab. Cardiovas. 21(5), 315–322 (2011)
S. Qu, Z. Li, Q. Jia, Detection of purine metabolite uric acid with picolinic-acid-functionalized metal–organic frameworks. ACS Appl. Mater. Interfaces 11(37), 34196–34202 (2019)
I. Kim, Y.I. Kim, S.W. Lee, H.G. Jung, G. Lee, D.S. Yoon, Highly permselective uric acid detection using kidney cell membrane-functionalized enzymatic biosensors. Biosens. Bioelectron. 190, 113411 (2021)
Y. Li, S. Li, M. Bao, L. Zhang, C. Carraro, R. Maboudian, A. Liu, W. Wei, Y. Zhang, S. Liu, Pd nanoclusters confined in ZIF-8 matrixes for fluorescent detection of glucose and cholesterol. ACS Appl. Nano Mater. 4(9), 9132–9142 (2021)
Y. Huang, Y. Gu, X. Liu, T. Deng, S. Dai, J. Qu, G. Yang, L. Qu, Reusable ring-like Fe3O4/Au nanozymes with enhanced peroxidase-like activities for colorimetric-SERS dual-mode sensing of biomolecules in human blood. Biosens. Bioelectron. 209, 114253 (2022)
J. Hassanzadeh, A. Khataee, Ultrasensitive chemiluminescent biosensor for the detection of cholesterol based on synergetic peroxidase-like activity of MoS2 and graphene quantum dots. Talanta 178, 992–1000 (2018)
V. Román-Pizarro, M. Ramírez-Gutiérrez, A. Gómez-Hens, J.M. Fernández-Romero, Usefulness of magnetically-controlled MNPs-enzymes microreactors for the fluorimetric determination of total cholesterol in serum. Talanta 208, 120426 (2020)
H.C. Chang, J.A. Ho, Gold nanocluster-assisted fluorescent detection for hydrogen peroxide and cholesterol based on the inner filter effect of gold nanoparticles. Anal. Chem. 87(20), 10362–10367 (2015)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 82172829, 21605114).
Funding
National Natural Science Foundation of China, 82172829, Hong-Tao Zhao, 21605114, Xian-Hua Wang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, HT., Lang, JY., Wang, Z. et al. Bioconjugation of nanozyme and natural enzyme for ultrasensitive detection of cholesterol. ANAL. SCI. 39, 503–515 (2023). https://doi.org/10.1007/s44211-022-00258-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-022-00258-5