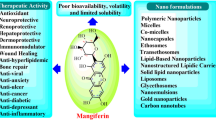
Abstract
Nutraceuticals have recently gained interest owing to their valuable contribution in the treatment of several diseases, with high safety margin and low incidence of side effects. However, their efficacy is limited by some challenges, namely poor solubility, low permeability, and, consequently, low bioavailability. Delivery carriers have proven that they can overcome almost all the aforementioned limitations, leading to improvement in the pharmacological efficacy of nutraceuticals. Among the promising nutraceuticals that have currently evoked considerable interest is mangiferin from mango tree, which is a polyphenol exhibiting many favorable pharmacological actions, but unfortunately suffers from poor aqueous solubility and other limitations that lower its bioavailability and halter its efficacy. This review summarizes the pharmacological actions of mangiferin and provides an insight on how delivery carriers for mangiferin (lipidic, vesicular, polymeric, inorganic, and protein nanoparticles, as well as complexes) can overcome its pharmaceutical challenges, hence reflecting on its improved therapeutic effects in treatment of different diseases.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, there has been a great emphasis on small molecules sourced from different plants, owing to their long-term medicinal activities (Fadel et al. 2018; El-Gogary et al. 2019). Among these molecules is the xanthonoid compound mangiferin (1), which is a natural C-glucoside xanthone (2-C-β-d-gluco-pyranosyl-1,3,6,7-tetrahydroxyxanthone) (Khurana et al. 2016a). It is found in many plant species; however, it is primarily obtained from the leaves, stem barks, roots, and fruits of mango tree Mangifera indica L., Anacardiaceae (Nunez Selles et al. 2016; Khurana et al. 2017a).

Mangiferin (1) provides protection against a wide range of physiological disorders (Telang et al. 2013), and it exhibits many pharmacological activities such as antidiabetic (Muruganandan et al. 2005); anti-inflammatory by acting as a potent inhibitor of cyclooxygenase-2 (COX2), interleukins (IL-6 and IL-8), X-linked inhibitor of apoptosis protein (XIAP), intercellular adhesion molecule 1 (ICAM1), chemokine receptor type 4 (CXCR4), tumor necrosis factor (TNF), and nuclear factor kappa B (NFκB) (Garcia-Rivera et al. 2011); analgesic (Ojewole 2005); immunomodulatory (Leiro et al. 2004); and antiviral (Wang et al. 2011). The antioxidant and free radical scavenging activity of mangiferin is mainly due to the polyphenolic groups in its structure (Saha et al. 2016). Interestingly, mangiferin’s antitumor activity has been recently disclosed, where it has shown both direct and auxiliary roles in oncotherapy (Du et al. 2018), as proven by the in vitro cell proliferation/toxicity assay delineating that 1 inhibited the human promyelocytic leukemia cell HL-60 growth in a dose- and time-dependent manner. Mangiferin efficaciously triggers G2/M phase cell cycle arrest by downregulation of the cyclin-dependent kinase 1 (CDK1)/cyclin B1 signaling pathway (Peng et al. 2015). This was proven by RT-PCR results, showing that Chk1 and cdc25c mRNA expression was declined after treatment with mangiferin at a concentration 200 mM, while inhibiting ATR phosphorylation starting at 24 h of treatment. Further investigations on the protein expression levels of cdc2 and cyclin B1 were carried out, and findings showed that after treatment with mangiferin, the expression levels of cdc2 and cyclin B1 were decreased, resulting in A549 human lung carcinoma cell proliferation inhibition. In addition, mangiferin inhibits the transcriptional activity of NF-κB and induces apoptosis of tumor cells by suppressing the phosphorylation of NF-κB kinase, indicating that it induces apoptosis via suppression of the PKC-NF-κB signaling pathway in A549 human lung carcinoma cells, which may function as an effective strategy in cancer treatment (Shi et al. 2016).
Multiple signaling pathways are implicated in the anticancer activity of mangiferin, including nuclear NF-κB signaling and COX-2 protein expression (Telang et al. 2013; Khurana et al. 2016b). Table S1 demonstrates the pharmacological actions of mangiferin (1), as well as the related mechanisms.
Despite the pharmacological merits of mangiferin, it faces several problems which adversely affect its therapeutic efficacy. It is classified in the biopharmaceutics classification system as a class IV compound (low solubility and permeability) due to its low aqueous solubility (Ma et al. 2014), which results in a poor oral bioavailability and intestinal permeability (Nunez Selles et al. 2016). Moreover, mangiferin exhibits high hepatic first-pass metabolism (Tian et al. 2016a) and high P-gp efflux (Louisa et al. 2014). All of the aforementioned result in low mangiferin bioavailability (about 1.2%) (Liu et al. 2012; Khurana et al. 2017a), hence restricting its eventual clinical use (Tian et al. 2016b).
Recently, several studies have focused on the improvement of mangiferin bioavailability through carrier-based delivery, which increases its solubility and membrane permeability. A summary of these approaches is provided in the next section. Table S2 summarizes these delivery carriers.
Search Strategy
A systematic search in the literature was performed in Google Scholar, PubMed, Scopus, and Web of Science databases using all available MeSH terms for mangferin, physicochemical properties, pharmacological properties, pharmacokinetics, solubility, bioavailability, and uses of mangiferin. The article search time was from inception to the year 2022.
Discussion
Physicochemical Properties
Mangiferin is a major compound, constituting 41% of the aqueous extract of Mangifera indica L. plant (De Los Monteros-Zuñiga et al. 2016). It is also found in other plant groups such as Apiaceae, Celastraceae, Gentianaceae, and Zingiberaceae. The presence of xanthinol core structures with polyhydroxy and C-hydroxyl linkage in mangiferin structure confers the ability to perform numerous biological functions (Jamwal et al. 2022). The structure of 1 has two aromatic rings, non-aromatic secondary hydroxyl groups, one lactonic carbonyl group, and one primary glycosidic hydroxyl group (Jyotshna et al. 2016). Mangiferin has another isomer existing as isomangiferin, but it has no chiral structure. Moreover, in nature, it has two similar structures: homomangiferin and neomangiferin (Mei et al. 2021a). It is a yellow crystalline solid with molecular weight 422 g/mol and melting point at 274 °C (Khurana et al. 2017a), and log P 2.73 (Khurana et al. 2016b; Ochocka et al. 2017). It is sparingly soluble in water (1.44 mg/ml) (Acosta et al. 2016; Khurana et al. 2016a), slightly soluble in ethanol, sparingly soluble in methanol, and practically insoluble in diethyl ether, acetone, and n-hexane (Mei et al. 2021a).
Bioavailability, Metabolism, and Pharmacokinetics
Bioavailability
Mangiferin is categorized as a class IV drug under BCS classification (Ma et al. 2014). It has low solubility, poor transmembrane permeability, and intestinal metabolic instability resulting in poor oral bioavailability which limits its bioefficacy. The poor transmembrane absorption of mangiferin can be attributed to the C-glycosidic bond in its structure which is more stable than the O-glycosidic bond and not easily hydrolyzed. That’s why many approaches have been explored in order to increase both water-soluble and lipid-soluble properties of mangiferin, to achieve greater oral absorption and thus improve its bioavailability and therapeutic efficacy (Mei et al. 2021a). Mangiferin showed undetectable plasma concentration after oral administration of 50–500 mg/kg dose proving its poor oral bioavailability. Oral administration of a 30 mg/kg dose of mangiferin to rats only showed 1.15% bioavailability in comparison to intraperitoneal administration with higher absorption (Kammala 2015). Mangiferin was reported to be subjected to intensive hepatic first-pass metabolism, minimizing the fraction of the dose reaching the systemic circulation, which is a reason contributing to the low oral bioavailability in normal rats, leading to reduction of the efficacy of 1 (Gu et al. 2019). The relative permeability (Peff) and absorption rate constant of mangiferin were in the following order: duodenum > jejunum > colon > ileum, using an in situ intestinal perfusion model (Ma et al. 2014). Complexation of mangiferin with β-cyclodextrin (CD) is one of the approaches reported to increase its solubility and bioavailability (Jyotshna et al. 2016). Moreover, enhancing mangiferin’s bioavailability can be done physically by encapsulating it in nano-based carrier systems (Mittal et al. 2020).
Metabolism
Mangiferin and its aglycone were found to be extensively metabolized in vivo, as proven by several studies on experimental models (Saha et al. 2016). The first step in mangiferin’s metabolism is deglycosylation to the aglycone; norathyriol, with apparent bioavailability of 30.4% that is much higher than that of mangiferin (Mei et al. 2021a). This is followed by the action of gut microbiome phase II enzymes that work on conjugating norathyriol to produce norathyriol-bound metabolites (Guo et al. 2018). The possible metabolic biotransformation reactions of mangiferin include deglycosylation, dehydroxylation, methylation, glycosylation, glucuronidation, and sulfation, resulting in around thirty-three identified metabolites of 1 in a rodent feeding study (Mei et al. 2021a). It was found that mangiferin and its metabolites were distributed within an hour in various human tissues and organs as the stomach, liver, small intestine, heart, brain, spleen, and lung after a single oral dose. The identified metabolites are mainly excreted in urine (Saha et al. 2016).
Pharmacokinetics
Mangiferin was found to follow nonlinear pharmacokinetics, represented by the two-compartmental model. This nonlinearity is mainly because of the saturated binding and metabolism. By studying the pharmacokinetics of mangiferin in human volunteers after oral administration of an amount of 0.9 g, it was found to follow nonlinear pharmacokinetics with maximum plasma concentration Cmax of 38.64 ± 6.75 ng/ml in 1 h and elimination half-life (t1/2) of 7.85 ± 1.72 h. Moreover, it was found that the intestinal flora helps in the absorption and metabolism of mangiferin, as illustrated by a comparative study of its metabolism and pharmacokinetics in conventional rats, pseudo-germ-free rats, and streptozotocin-induced diabetic rats (Jyotshna et al. 2016).
Toxicology
Being a compound of natural origin, mangiferin is generally considered a non-toxic compound. Oral administration of a dose of 0.9 g of mangiferin showed no toxicity (Hou et al. 2012). Mangiferin’s safety as well as its beneficial effect in improving cellular function was reported by several studies (Mei et al. 2021b). The vascular protection effect of mangiferin in a range from 0.1 to 100 μM was proven without any cytotoxicity (Wisutthathum et al. 2019). Also bone marrow erythrocytes showed no genotoxicity after oral application of 2 g/kg of mangiferin to NMRI mice (Rodeiro et al. 2012). Oral administration of mango leaf extract (60% mangiferin) for three months period at a dose of 2 g/kg to rats showed no mortality or toxic effects (Reddeman et al. 2019). Moreover, orally administrated mangiferin nanocapsules for 24 days did not show any toxic signs to Wistar rats, and was considered safe (Do Carmo et al. 2019). Although it was reported that mangiferin did not induce cytotoxicity, genotoxicity, or mutagenicity in studies involving blood peripheral lymphocytes and hepatocytes of rats, the oral administration of mangiferin in rodents showed low acute and sub-chronic toxicity in another study (Gold-Smith et al. 2016), which was attributed to the administration of high dose of 400 mg/kg of 1 with free radical scavenging activity, which might cause damage in the intracellular mitochondrial respiratory chain (Saha et al. 2016). However, the oral administration of mangiferin has a wide safety margin.
Carrier-Based Delivery Systems
Lipid-Based Carriers
Lipid-based carriers have been proven safe and efficient for the formulation of nutraceuticals (Muller et al. 2002; Hatem et al. 2018a, 2018b) due to their high degree of biocompatibility. They were reported to improve the solubility and bioavailability of poorly water-soluble drugs, and provide site-specific and time-controlled delivery of drugs (Brigger et al. 2002; Shrestha et al. 2014). Among the most prominent lipidic carriers are solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), and nanoemulsions.
Nanostructured Lipid Carriers
Nanostructured lipid carriers are drug carriers composed of biodegradable solid lipid matrix and liquid lipid core matrix, which improve the stability of drugs during storage, increase their solubility, prolong their half-lives, and improve their permeability and bioavailability (Fang et al. 2013). Although NLCs offer all of these advantages, their mass production is still challenging (Tapeinosa et al. 2017). In a study by Liu et al. (2012), NLCs of mangiferin were successfully prepared for improving its solubility and ocular bioavailability, using a mixture of two solid lipids (glyceryl monostearate and gelucire 44/14) and miglyol 812, as liquid lipid. A mean particle size below 100 nm and entrapment efficiency reaching 88% were achieved, with sustained release (87%) over a period of 12 h. Mangiferin-NLCs increased the in vitro corneal permeation by 2.61-folds, and they were non-irritant to the rabbits’ eyes, with a prolonged residence time on the corneal surface and in the conjunctival sac. Similar results were obtained by Santonocito et al. (2022), who reported that 1-NLCs prepared using the solid lipid compritol 888 ATO and liquid lipid miglyol 812 enhanced the antioxidant properties of mangiferin, and were non-irritant to ocular tissues. Furthermore, a 1:1 phospholipidic complex of mangiferin was prepared for enhanced water solubility and oil-water partition coefficient, and then further loaded in NLCs composed of compritol 888 as solid lipid and labrafil M 2125 as liquid lipid (Khurana et al. 2017b). High encapsulation efficiency reaching 87% was obtained, with sustained release potential up to 10 h. Furthermore, the aforementioned NLCs better inhibited the P-gp efflux activity on Caco-2 cells compared to mangiferin alone, and led to 4.55- and 1.98-fold increase in the maximum plasma concentration (Cmax) and area under the curve (AUC) respectively, and 3-fold reduction in the time for maximum absorption (tmax) compared to mangiferin alone.
Nanoemulsions
Nanoemulsions consist of a dispersion of droplets of one liquid within another immiscible liquid, with the aid of tensioactive substances such as surfactants (Ismail et al. 2020). They represent versatile carriers for delivery of lipophilic, hydrophilic, and amphiphilic molecules, thus improving the bioavailability of poorly absorbed herbs actives/extracts (Mohammad et al. 2017). However, nanoemulsions have some limitations such as unavailability of surfactants and cosurfactants, and poor stability owing to Ostwald’s ripening; which is a process where growth of a larger droplet occurs when smaller droplets disintegrate and deposit on the larger ones (Naseema et al. 2021). Mangiferin oil-in-water nanoemulsion hyaluronate gels were prepared for skin inflammatory disorders by Pleguezuelos-Villaa et al. (2019), and it was proven that these nanoemulsions increased the solubility of mangiferin with enhancement of its permeation to epidermis and dermis.
Self-microemulsifying drug delivery system (SMEDDS), which is a homogeneous stable mixture of drug with natural or synthetic oils, surfactant, and co-surfactant that upon mild agitation of the gastrointestinal tract can easily form emulsion (oil-in-water droplets) with a diameter size of less than 50 nm without the dissolution process, was loaded with phospholipidic complex of mangiferin (Xuan et al. 2012), using isopropyl myristate, Cremphor EL35, and Labrasol. The prepared mangiferin-SMEDDS were found to improve the dissolution of the active principle.
Water-in-Oil-in-Water Double Emulsion
The double emulsion system is composed of small droplets of water encapsulated into larger oil droplets, which are then dispersed into the aqueous phase that requires the use of both hydrophobic and hydrophilic emulsifiers for stabilization. It is considered an effective approach for encapsulating polyphenols as mangiferin because their double-layer structure acts as a protective barrier against environmental factors such as light, oxygen, moisture, and temperature (Xing et al. 2022). However, one of the major problems of the double emulsions water-in-oil-in-water emulsion is their thermodynamic instability manifested by creaming, leading to coalescence and separation (Benna-Zayani et al. 2007).
Mangiferin (1) was successfully encapsulated in water-in-oil-in-water double emulsion with the use of 3% of the hydrophobic emulsifier polyglycerol polyricinoleate and 3% of hydrophilic emulsifier calcium caseinate for stabilization. The system presented a satisfactory entrapment efficiency for 1 reaching 95.31%. The confocal laser scanning microscope images illustrated the presence of many small water droplets encapsulated within the large oil droplets, which is a typical structure of water-in-oil-in-water emulsion, with a particle size of 49.90 ± 8.34 μm and negative zeta potential ranging from −13.69 mV to −17.06 mV due to the negatively charged calcium caseinate. The rheological assessment of the prepared systems showed high apparent viscosity and gel state with non-Newtonian flow properties (shear-thinning behavior). The prepared systems were successful in encapsulating mangiferin with high stability required in a variety of food, cosmetic, and pharmaceutical applications (Xing et al. 2022).
Vesicular-Based Carriers
Transfersomes
Phospholipid vesicles are considered among the most valuable and versatile systems, due to their biocompatibility and similarity to body components (Castangia et al. 2015; Agiba et al. 2018; Nasr and Wahdan 2019) and are particularly used for skin delivery. Transfersomes are elastic phospholipidic vesicles, containing an edge activator which imparts flexibility to the vesicles, and allows them to be used for both topical and transdermal drug delivery (Cevc et al. 1998). Although the advantages of transfersomes, they exhibit chemical instability due to their tendency for oxidative degradation, and the cost of pure natural phospholipids (Opatha et al. 2020). Transfersomes are expected to improve the topical delivery of mangiferin, since the conventional cream or gel exhibit low bioavailability for mangiferin at the skin level, as they hardly cross the stratum corneum (Telang et al. 2013).
Therefore, in an attempt to enhance skin absorption and bioavailability of mangiferin, Allaw et al. (2020) incorporated it in transfersomes containing Tween 80 as the edge activator, modified with glycerol and propylene glycol in combination, in the presence or absence of mucin, yielding either glycoltransfersomes or mucin-glycoltransfersomes, respectively. The prepared transfersomes were less than 300 nm in size, with high entrapment efficiency for mangiferin, and it was reported that its accumulation in the epidermis and dermis was enhanced by incorporating glycols to the transfersomes. The scratch wound healing assay proved the ability of mangiferin-loaded glycoltransfersomes, mucin-transfersomes, and mucin-glycoltransfersomes to promote complete wound healing at 32 h. Moreover, the vesicles were cytocompatible, capable of protecting fibroblasts from oxidative stress and mouse skin from chemically induced injury, and they reduced inflammatory infiltration and promoted skin regeneration in mice.
Herbosomes
The term “herbosomes” refers to lipid vesicular structures formed by complexation of phospholipids with a standardized plant extract or active herbal ingredients, allowing them to cross the biological membranes and increase their absorption and bioavailability (Bombardelli et al. 1994).
Mangiferin herbosomes or mangiferosomes were prepared by dissolving it and soya phosphatidylcholine (SPC) together in 1:1 molar ratio in dichloromethane followed by solvent evaporation, and dispersion with cholesterol in phosphate-buffered saline (pH 7.4) (Jain et al. 2013). Mangiferin herbosomes were in the size range of 0.2–2 μm, and the ex vivo study on everted small intestine sac technique showed enhanced absorption and more cumulative release as compared to the plain drug. In addition, the antioxidant potential and the in vivo hepatoprotective activity of mangiferin were significantly enhanced when encapsulated in the herbosomal form, manifested by a decrease in the levels of the liver enzymes serum glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), and alkaline phosphatase (ALP).
Glycethosomes
Glycethosomes are phospholipid vesicles prepared by hydrating the phospholipid with a mixture of water, glycerol, and ethanol, in which glycerol is added for its moisturizing and cosolvent properties, and ethanol is added as an edge activator (Perez et al. 2016; Angelova-Fischer et al. 2018). However, the high viscosity may delay the passage of vesicles from the formulation to the skin (Zhang et al. 2022). Glycethosomes loaded with mangiferin were prepared by Pleguezuelos-Villaa et al. (2020) as a new potential adjuvant for the treatment of psoriasis. Small-sized mangiferin glycethosomes were obtained (140 nm), and the skin drug deposition experiment delineated that the application of glycethosomes loaded with mangiferin led to 9-fold increase in the deposited amount of mangiferin compared to the dispersion containing the same amount of drug. Moreover, the in vitro cell viability study showed that mangiferin glycethosomes were able to counteract the oxidative stress induced in fibroblasts using hydrogen peroxide. Hydrogen peroxide was used to stress the cells, followed by treatment with mangiferin glycethosomes. The stressed cells with hydrogen peroxide showed very low cell viability reaching ~40%. The oxidative stress was slightly counteracted by the addition of mangiferin dispersion that leveled up the cell viability to ~70%. However, the best oxidative stress cell protection results were obtained by the use of mangiferin-loaded glycethosomes, and the viability of the cells reached ~100%. Furthermore, mangiferin glycethosomes showed significant inhibition of edema and myeloperoxidase (MPO) activity in mice exposed to 12-O-tetradecanoylphorbol-3-acetate (TPA). Histological analysis showed that the topical application of mangiferin glycethosomes was able to reduce the pathological effect induced by TPA in comparison with the mangiferin dispersion. This study highlighted the remarkable therapeutic potential of mangiferin glycethosomes for treatment of psoriasis or other skin disorders.
Ethosomes and Transethosomes
Ethosomes are biocompatible nanovesicular systems that are closely related to liposomes in composition (phosphatidylcholine and water). However, they differ from classic liposomes in the presence of ethanol (20–45%) which improves the vesicle stability and the entrapment capacity of lipophilic drugs, resulting in enhancement in the vesicle penetration potential. Transethosomes are a new generation of ethosomes, composed also of phosphatidylcholine, ethanol, and water, with surfactants, which act as edge activators. The presence of surfactants in transethosomes results in more flexible vesicles than ethosomes. Owing to their remarkable transdermal potential, both ethosomes and transethosomes can be used for the cutaneous delivery of specific drugs in the treatment of several skin pathologies (Sguizzato et al. 2021). Although ethosomes and transethosomes provide numerous advantages, few disadvantages of ethosomes were reported like poor yield and coalescence (Ramakrishna et al. 2014).
Ethosomes and transethosomes loaded with mangiferin were prepared successfully, showing spherical and ovoid multilamellar vesicles with size ranging from 86.41 to 189.8 nm. Using transmission electron microscopy, the uptake of the prepared mangiferin-loaded vesicles in keratinocytes was obvious. The antioxidant and anti-inflammatory effects of mangiferin vesicles against the cutaneous tissue damage induced by cigarette smoke were significantly proved. This was demonstrated by the significant prevention of the expression of the key enzymes involved in cellular oxidative defense (interleukin-6, a pro-inflammatory cytokine and Heme Oxygenase 1 enzyme) in HaCaT cells treated with mangiferin vesicles prior to cigarette smoke (Sguizzato et al. 2021).
Polymer-Based Carriers
Polymeric carriers are solid particles mostly composed of biodegradable and biocompatible polymers of synthetic or natural origin, in which the drug can be encapsulated within the carrier, physically adsorbed on the surface of the carrier, or chemically linked to the surface. Mangiferin was reported to be loaded in different polymeric systems (particularly nanosized ones), since they have many advantages such as the small size, stability during storage, water solubility, biodegradability, safety, and long shelf life. However, one of their major challenges is the difficulty for scaling-up.
Polymeric Nanoparticles and Microparticles
Polymeric nanoparticles and microparticles are nanosized/microsized matrix spheres, respectively, which can be prepared using several polymers (Abd-Allah et al. 2020). They have been widely used for drug delivery because they can be used for site-specific targeting, by attaching targeting moieties to particles’ surface, and modulation of the controlled release and particle degradation characteristics can be readily done by variation of matrix components (Nikam et al. 2014). Despite the advantages of polymeric nanoparticles and microparticles, their use has some limitations that are related to their shape, wide size distribution, and possibility of particle-particle aggregation (Begines et al. 2020).
Chitosan is an attractive polymer for drug delivery, owing to its safety, biocompatibility, and biodegradability (Abd-Allah et al. 2020). In an attempt to make use of chitosan in the delivery of mangiferin, Yusri et al. (2020) prepared mangiferin carboxymethylchitosan nanoparticles, and evaluated their effectiveness against osteosarcoma MG63 cells. Results revealed that the prepared chitosan nanoparticles were of small size (around 200 nm), and they exhibited a significant reduction in the growth of osteosarcoma MG-63 cells, with IC50 values ranging between 7.8 and 15.6 μg/ml. Similar results were obtained by Samadarsi and Dutta (2020), who prepared mangiferin chitosan nanoparticles using 1% (w/v) chitosan solution and sodium tripolyphosphate (TPP) and proved its antioxidant effect by preventing the induction of cytotoxicity induced by sodium fluoride (NaF) and maintaining the level of intracellular antioxidant enzyme in the cells.
Poly (lactic-co-glycolic acid) (PLGA) is another promising polymer characterized by its biodegradability (Nasr et al. 2011). PLGA nanoparticles of mangiferin were prepared by Razura-Carmona et al. (2019) in an attempt to overcome the problems of low solubility, poor bioavailability, as well as degradation of 1. The prepared mangiferin PLGA nanoparticles showed mean particle size of 177 nm, a high entrapment efficiency for 1 reaching 97%, and controlled release while resisting its gastric digestion.
Pectin is a non-toxic polysaccharide polymer that is found in the cell wall of most plants and is not digested by gastric or intestinal enzymes. Pectin forms water-insoluble complexes with several drugs and may be a useful additive for sustained-release preparations (Naggar et al. 1992; Aydin and Akbuĝa 1996). Therefore, to prepare controlled release formulation for 1, De Souza et al. (2009) reported its encapsulation in pectin spheres which were further coated with chitosan. The resultant beads (660–670 μm) were able to encapsulate 1 at high percent (58%), with controlled release potential in the stomach and intestine.
Moreover, poly (ε-caprolactone)-poly (ethyleneglycol)-poly (ε-caprolactone) (PCL-PEG-PCL, PCEC) copolymers were converted to magnetic nanoparticles, and loaded with mangiferin, utilizing an external magnetic field for targeting the drug in cancer chemotherapy (Xiao et al. 2021). The encapsulated magnetic/polymeric nanoparticles displayed higher cytotoxicity on several cancer cell lines such as MCF-7, A549, and Hela cells.
Polymeric Nanocapsules
Polymeric nanocapsules are spherical nanosized particles which consist of a rigid polymeric shell acting as a protecting barrier, surrounding an oil core that acts as a drug reservoir (Nasr and Abdel-Hamid 2015; Aldalaen et al. 2019). The solid/oil core of nanocapsules can effectively increase drug-loading efficiency, and the polymeric shell protects the encapsulated drug from the tissue environment, so avoiding the degradation or burst release induced by pH, temperature, or enzymes. Moreover, nanocapsules can be used for targeted drug delivery by functionalizing the polymeric shell by moieties that can interact with targeted biomolecules. Despite the merits of nanocapsules, they tend to be unstable and aggregate in water suspension, thereby inducing leaking of the encapsulated drug (Deng et al. 2020). Mangiferin nanocapsules of 95-nm size were prepared by Moura et al. (2014) using Eudragit S100 as the polymer, exhibiting controlled release for 1, and were delineated as promising for improving its bioavailability.
Polymeric Mixed Micelles
Polymeric mixed micelles are composed of two or more distinct amphiphilic polymers which self-assemble into nanostructures with sizes ranging between 20 and 200 nm. They offer various advantages over single polymeric micelles such as improvement in the thermodynamic and kinetic stabilities, accurate size control, and enhanced drug loading capacity (Attia et al. 2011). However, a reported disadvantage of polymeric micelles is their small loading capacity (Ahmad et al. 2014).
Khurana et al. (2017b, 2018) reported the preparation of phospholipidic micellar system of mangiferin, for increasing its oral bioavailability. The prepared mangiferin phospholipidic micellar systems ranged in size between 15 and 60 nm, which improved the dissolution rate of the drug compared to the suspension, with concomitant enhanced intestinal permeability and absorption using everted gut sac. The prepared system also enhanced the therapeutic efficacy of 1 on the breast cancer cell line MCF-7, with lower P-gp drug efflux. Moreover, the values of Cmax, AUC, and absorption constant (Ka) were nearly increased by 4.22-, 2.96-, and 3.59-folds, respectively, suggesting the superiority of the phospholipidic mixed micellar system to the mangiferin alone.
Mangiferin was also encapsulated in the mixed micellar system composed of Pluronic F127 (F127), Pluronic P123 (P123), and d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) copolymers to improve its oral bioavailability associated with its poor aqueous solubility (Thanitwatthanasak et al. 2019). The prepared mangiferin mixed micelles ranged in size from 12 to 22 nm, with high stability against aggregation, and a high entrapment efficiency reaching 95%. Moreover, the mixed micelles increased the dissolution of 1, making them suitable for oral delivery.
Inorganic Carriers
Mesoporous Silica
Mesoporous silica is a synthetic material that has attracted considerable attention owing to its large surface area, well-defined pore structure, thick walls, thermal and hydrothermal stability, safety, and high biocompatibility (Zhao et al. 1998). Under appropriate conditions, its pore architecture enables it to be used as a pharmaceutical carrier for a variety of drugs, to control their release kinetics from the matrix (Silva et al. 2017). Mesoporous silica was also reported to enhance the bioavailability of drugs with low solubility in water, because the pore size creates large surfaces allowing the adsorption of organic molecules and their delivery (Mellaerts et al. 2011). However, mesoporous silica faces some challenges such as difficulty in preparation of a well-ordered structure, having scattered size distribution, and formation of stable-colloidal suspension (Jafari et al. 2019).
For mangiferin (1) delivery, Silva et al. (2017) prepared mesoporous silica with tubular pores of 6 nm in size, which accelerated the dissolution and diffusion of 1 from the mesoporous channels to the medium and enhancing its solubility by almost 2.5-folds.
Gold Nanoparticles
Gold nanoparticles are metallic particles which are non-toxic, biocompatible, with macroscopic quantum tunneling effect, distinct surface effect, ultra-small size, and surface plasmon resonance (SPR) bands (Hainfeld et al. 2005; Kumar et al. 2013). Despite the exciting data obtained in laboratory studies, some challenges facing gold nanoparticles include inability to evade the clearance of the mononuclear phagocytic system (MPS) or renal excretion (Bai et al. 2020). Mangiferin was able to chemically reduce the gold precursor to gold nanoparticles of particle size 35–55 nm (Al-Yasiri et al. 2017), and they were successfully able to target the laminin receptors on the prostate tumor, suggesting their promising use in cancer treatment.
Carbon Nanotubes
Carbon nanotubes are nanomaterials which are formed of carbon atom sheets arranged into a honeycomb-like lattice rolled into a tube shape with a diameter of only few nanometers (Vines et al. 2019). Despite their advantages in drug delivery, some limiting factors for carbon nanotubes in nanomedical applications include the existence of impurities, insolubility, lack of uniformity in shape and structure, and their tendency to aggregate (Porwa et al. 2017). Polyethylene glycol–linked conjugate of carbon nanotubes with mangiferin was synthesized to deliver 1 to cancer cells using carbon nanotubes (CNTs), and to enhance its biological residence due to polyethylene glycol (PEG) coating (Harsha et al. 2019). The resultant system was 70 nm in size, with higher percent released of 1 in the acidic pH of the tumor, and it decreased the degree of protein binding of mangiferin, hence ensuring better availability of the drug. Concerning the in vitro cytotoxicity studies carried out on U-87 glioblastoma cell line, there was almost 1.28-fold decrease in the IC50 value, indicating an increase in the efficacy of 1 at low doses after conjugation with CNT-PEG. The pharmacokinetic study showed that bioavailability of mangiferin was increased by almost 4 times relative to the pure drug.
Protein Nanoparticles
Proteins exhibit unique functions and properties in biological materials and manufacturing fields and can be used in the production of nanoparticles. As a drug delivery system, protein nanoparticles offer numerous advantages, such as biodegradability, stability, surface modification of particles, and ease of particle size control. Moreover, protein nanoparticles can be transmitted through the cell via endocytosis due to their small size (Hong et al. 2020). Although protein nanoparticles have various advantages in drug delivery, they have some limitations such as lack of reproducibility, and variation in product characteristics at different times (batch to batch variation) during mass production (Kianfar 2021).
β-Lactoglobulin (β-LG) is a lipocalin protein with molecular weight of 18.4 kDa and small size of 3.6 nm and is resistant to stomach digestion by pepsin while being digested by trypsin in the small intestine (Samadarsi and Dutta 2019). Mangiferin-β-LG nanoparticles were prepared using the physical cross-linker tripolyphosphate (TPP), displaying a size of 89 nm, and showing 80% release in simulated colonic fluid (SCF) within 8 h and only 9% release in simulated gastric fluid (SGF) (Samadarsi and Dutta 2019). This delineates β-LG nanoparticles as a promising system for the oral site-directed targeted delivery of 1. Furthermore, the aforementioned mangiferin-β-LG nanoparticles were modified by chitosan coating by the same group (Samadarsi et al. 2020) for milk fortification, yielding particles of 135 nm in size and high entrapment for 1 of almost 92%, and high free radical scavenging activity and inhibition of both lipid peroxidation and protein oxidation.
Complexes
The use of complexes is among the strategies applied to increase drug solubilization, increase drug stability, reduce irritation to the human gastrointestinal tract, and mask unpleasant taste. On the other hand, using complexation in drug delivery has showed some disadvantages as the potential toxicity and quality control issues related to the presence of the ligand may add complication and cost to the development process (Wen et al. 2015).
In order to increase the solubility and membrane permeability of mangiferin, it was complexed with phospholipids, yielding 1.4 times higher aqueous solubility, and 30 times higher solubility in the n-octanol phase compared to crude 1, with increased intestinal membrane permeability in vivo (Ma et al. 2014), and higher average Cmax. Mangiferin-phospholipidic complex further showed an enhancement in efficacy in preventing the adverse effects caused by carbon tetrachloride intoxication in rats, with significant improvement in the bioavailability, and reduced clearance (Bhattacharyya et al. 2013). When the mangiferin-phospholipidic complex was processed into the formation of self-assembled soft nanoparticles, they displayed higher permeability than the native complex across the everted rat intestinal membrane, correlating with better hepatoprotective activity (Telange et al. 2020).
Mangiferin was also complexed with polyamine-modified β-cyclodextrin (CD), which is a polysaccharide composed of seven d-glucose monomers linked by α-1,4-glucose bonds having a hydrophobic interior cavity with a hydrophilic exterior, yielding an inclusion complex. The complex resulted in significantly enhanced water solubility of mangiferin, with improvement of its cytotoxicity compared to native compound 1 (Liang et al. 2019).
Perspectives and Future Directions
As evident from the preclinical studies conducted on mangiferin (1) nanoparticles, it was demonstrated that the therapeutic efficacy and pharmacokinetic parameters of 1 were significantly enhanced following the administration within nanoparticles. Therefore, future research studies need to focus on conducting clinical trials on mangiferin nanoparticles, in order to translate the preclinical findings into tangible clinical applications.
Conclusions
In an attempt to improve the water solubility and bioavailability of the natural molecule mangiferin, several delivery systems were summarized in this review. Findings revealed that the use of pharmaceutical carriers as delivery systems for mangiferin has shown a noticeable improvement in mangiferin’s solubility, permeability, bioavailability, and therapeutic activity. This is expected to ensure an improved health outcome for the patients upon potential clinical applications especially when treatment of a chronic condition with prolonged medical attention is needed, for example, in antidiabetic therapy.
References
Abd-Allah H, Abdel-Aziz RT, Nasr M (2020) Chitosan nanoparticles making their way to clinical practice: a feasibility study on their topical use for acne treatment. Int J Biol Macromol 156:262–270. https://doi.org/10.1016/j.ijbiomac.2020.04.040
Acosta J, Sevilla I, Salomón S, Nuevas L, Romero A, Amaro D (2016) Determination of mangiferin solubility in solvents used in the biopharmaceutical industry. J Pharm Pharmacogn Res 4:49–53
Agiba AM, Nasr M, Aabdel-Hamid S, Eldin AB, Geneidi AS (2018) Enhancing the intestinal permeation of the chondroprotective nutraceuticals glucosamine sulphate and chondroitin sulphate using conventional and modified liposomes. Curr Drug Deliv 15:907–916. https://doi.org/10.2174/1567201815666180123100148
Ahmad Z, Shah A, Siddiq M, Heinz-Bernhard Kraatz H-B (2014) Polymeric micelles as drug delivery vehicles. RSC Adv 4:17028–17038. https://doi.org/10.1039/c3ra47370h
Aldalaen S, El-Gogary RI, Nasr M (2019) Fabrication of rosuvastatin-loaded polymeric nanocapsules: a promising modality for treating hepatic cancer delineated by apoptotic and cell cycle arrest assessment. Drug Dev Ind Pharm 45:55–62. https://doi.org/10.1080/03639045.2018.1515221
Allaw M, Pleguezuelos-Villa M, Manca ML, Caddeo C, Aroffu M, Nacher A, Diez-Sales O, Saurí R, Ferrer EE, Fadda AM, Manconi M (2020) Innovative strategies to treat skin wounds with mangiferin: fabrication of transfersomes modified with glycols and mucin. Nanomedicine 15:1671–1685. https://doi.org/10.2217/nnm-2020-0116
Al-Yasiri AY, Khoobchandani M, Cutler CS, Watkinson L, Carmack T, Smith CJ, Kuchuk M, Loyalka SK, Lugão AB, Katti KV (2017) Mangiferin functionalized radioactive gold nanoparticles (MGF-198AuNPs) in prostate tumor therapy: green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalton Trans 46:14561–14571. https://doi.org/10.1039/c7dt00383h
Andreu GP, Delgado R, Velho JA, Curti C, Vercesi AE (2005) Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur J Pharmacol 513:47–55. https://doi.org/10.1016/j.ejphar.2005.03.007
Angelova-Fischer I, Rippke F, Richter D, Filbry A, Arrowitz C, Weber T, Fischer TW, Zillikens D (2018) Stand-alone emollient treatment reduces flares after discontinuation of topical steroid treatment in atopic dermatitis: a double-blind, randomized, vehicle-controlled, left-right comparison study. Acta Derm Venereol 98:517–523. https://doi.org/10.2340/00015555-2882
Attia ABE, Ong ZY, Hedrick JL, Lee PP, Rachel Ee PL, Hammond PT, Yang YY (2011) Mixed micelles self-assembled from block copolymers for drug delivery. Curr Opin Colloid Interface Sci 16:182–194. https://doi.org/10.1016/j.cocis.2010.10.003
Aydin Z, Akbuĝa J (1996) Preparation and evaluation of pectin beads. Int J Pharm 137:133–136. https://doi.org/10.1016/0378-5173(95)04458-2
Bai X, Wang Y, Song Z, Feng Y, Chen Y, Zhang D, Feng L (2020) The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. Int J Mol Sci 21:2480. https://doi.org/10.3390/ijms21072480
Begines B, Ortiz T, Pérez-Aranda M, Martínez G, Merinero M, Argüelles-Arias F, Alcudia A (2020) Polymeric nanoparticles for drug delivery: recent developments and future prospects. J Nanomater 10:1403. https://doi.org/10.3390/nano10071403
Benna-Zayani M, Kbir-Ariguib N, Trabelsi-Ayadi M, Grossiord J-L (2007) Stabilisation of W/O/W double emulsion by polysaccharides as weak gels. Colloids Surf A Physicochem Eng Asp 316:46–54. https://doi.org/10.1016/j.colsurfa.2007.08.019
Bhattacharyya S, Ahmmed S, Saha BP, Mukherjee PK (2013) Soya phospholipid complex of mangiferin enhances its hepatoprotectivity by improving its bioavailability and pharmacokinetics. J Sci Food Agric 94:1380–1388. https://doi.org/10.1002/jsfa.6422
Bombardelli E, Cristoni A, Morazzoni P (1994) Phytosomes infunctional cosmetics. Fitoterapia 95:387–401. https://doi.org/10.3923/jps.2007.644.649
Brigger I, Dubernet C, Couvreur P (2002) Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 54:631–651. https://doi.org/10.1016/s0169-409x(02)00044-3
Campos-Esparza MR, Sánchez-Gómez MV, Matute C (2009) Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium 45:358–368. https://doi.org/10.1016/j.ceca.2008.12.007
Castangia I, Caddeo C, Manca ML, Casu L, Latorre AC, Díez-Sales O, Ruiz-Saurí A, Bacchetta G, Fadda AM, Manconi M (2015) Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohydr Polym 134:657–663. https://doi.org/10.1016/j.carbpol.2015.08.037
Cevc G, Gebauer D, Stieber J, Schätzlein A, Blume G (1998) Ultraflexible vesicles, transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim Biophys Acta Gen Subj 1368:201–215. https://doi.org/10.1016/s0005-2736(97)00177-6
De Los Monteros-Zuñiga AE, Izquierdo T, Quiñonez-Bastidas GN, Rocha-González HI, Godínez-Chaparro B (2016) Anti-allodynic effect of mangiferin in neuropathic rats: involvement of nitric oxide-cyclic GMP-ATP sensitive K+ channels pathway and serotoninergic system. Pharmacol Biochem Behav 150-151:190–197. https://doi.org/10.1016/j.pbb.2016.10.007
De Souza JRR, De Carvalho JIX, Trevisan MTS, De Paula RCM, Ricardo NMPS, Feitosa JPA (2009) Chitosan-coated pectin beads: characterization and in vitro release of mangiferin. Food Hydrocoll 23:2278–2286. https://doi.org/10.1016/j.foodhyd.2009.06.004
Deng S, Gigliobianco MR, Censi R, Martino PD (2020) Polymeric nanocapsules as nanotechnological alternative for drug delivery system: current status, challenges and opportunities. Nanomaterials 10:847–886. https://doi.org/10.3390/nano10050847
Do Carmo G, Dotto B, Borin DB, Garcez R, de Almeida VR, Baldissera MD, Schwertz CI, Mendes RE, Rech VC, Raffin R (2019) Toxicological evaluation of mangiferin-loaded polymeric nanocapsules in Wistar rats. Disciplinarum Scientia 20:231–242
Du S, Liu H, Lei T, Xie X, Wang H, He X, Tong R, Wang Y (2018) Mangiferin: an effective therapeutic agent against several disorders. Mol Med Rep 18:4775–4786. https://doi.org/10.3892/mmr.2018.9529
El-Gogary RI, Gaber SAA, Nasr M (2019) Polymeric nanocapsular baicalin: chemometric optimization, physicochemical characterization and mechanistic anticancer approaches on breast cancer cell lines. Sci Rep 9:11064. https://doi.org/10.1038/s41598-019-47586-7
Fadel M, Kassab K, Abd El Fadeel DA, Nasr M, El Ghoubary NM (2018) Comparative enhancement of curcumin cytotoxic photodynamic activity by nanoliposomes and gold nanoparticles with pharmacological appraisal in HepG2 cancer cells and Erluch solid tumor model. Drug Dev Ind Pharm 44:1809–1816. https://doi.org/10.1080/03639045.2018.1496451
Fang C, Al-Suwayeh SA, Fang J (2013) Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat Nanotechnol 7:41–55. https://doi.org/10.2174/1872210511307010041
Garcia-Rivera D, Delgado R, Bougarne N, Haegeman G, Berghe WV (2011) Gallic acidindanone and mangiferin xanthone are strong determinants of immunosuppressive anti-tumour effects of Mangifera indica L. bark in MDAMB231 breast cancer cells. Cancer Lett 305:21–31. https://doi.org/10.1016/j.canlet.2011.02.011
Garrido G, Gonzalez D, Delporte C, Backhouse N, Quintero G, Nunez-Selles AJ, Morales MA (2001) Analgesic and antiinflammatory effects of Mangifera indica L. extract (Vimang). Phytother Res 15:18–21. https://doi.org/10.1002/1099-1573(200102)15:1<18::aid-ptr676>3.0.co;2-r
Garrido-Suarez BB, Garrido G, Delgado R, Bosch F, Rabi MD (2010) A Mangifera indica L. extract could be used to treat neuropathic pain and implication of mangiferin. Molecules 15:9035–9045. https://doi.org/10.3390/molecules15129035
Giron MD, Sevillano N, Salto R, Haidour A, Manzano M, Jimenez ML, Rueda R, Lopez-Pedrosa JM (2009) Salacia oblonga extract increases glucose transporter 4-mediated glucose uptake in L6 rat myotubes: role of mangiferin. Clin Nutr 28:565574–565574. https://doi.org/10.1016/j.clnu.2009.04.018
Gold-Smith F, Fernandez A, Bishop K (2016) Mangiferin and cancer: mechanisms of action. Nutrients 8:396–421. https://doi.org/10.3390/nu8070396
Gu PC, Wang L, Han MN, Peng J, Shang JC, Pan YQ, Han WL (2019) Comparative pharmacokinetic study of mangiferin in normal and alloxan-induced diabetic rats after oral and intravenous administration by UPLC-MS/MS. Pharmacology 103:30–37. https://doi.org/10.1159/000493364
Guo X, Cheng M, Hu P, Shi Z, Chen S, Liu H, Shi H, Xu Z, Tian X, Huang C (2018) Absorption, metabolism, and pharmacokinetics profiles of norathyriol, an aglycone of mangiferin, in rats by HPLC-MS/MS. J Agric Food Chem 66:12227–12235. https://doi.org/10.1021/acs.jafc.8b03763
Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM (2005) Gold nanoparticles: a new X-ray contrast agent. Br J Radiol 79:248–253. https://doi.org/10.1259/bjr/13169882
Harsha PJ, Nagarani T, Manish K, Saurabh S, Anupama M, Rajneet K, Bhupinder S, Poonam N, Kaisar R (2019) A novel PEGylated carbon nanotube conjugated mangiferin: an explorative nanomedicine for brain cancer cells. J Drug Deliv Sci Technol 53:101186. https://doi.org/10.1016/j.jddst.2019.101186
Hatem S, Nasr M, Moftah NH, Ragai MH, Geneidi AS, Elkheshen SA (2018a) Clinical cosmeceutical repurposing of melatonin in androgenic alopecia using nanostructured lipid carriers prepared with antioxidant oils. Expert Opin Drug Deliv 15:927–935. https://doi.org/10.1080/17425247.2018.1517740
Hatem S, Nasr M, Moftah NH, Ragai MH, Geneidi AS, Elkheshen SA (2018b) Melatonin vitamin C-based nanovesicles for treatment of androgenic alopecia: design, characterization and clinical appraisal. Eur J Pharm Sci 122:246–253. https://doi.org/10.1016/j.ejps.2018.06.034
Hong S, Choi DW, Kim HN, Park CG, Lee W, Park HH (2020) Protein-based nanoparticles as drug delivery systems. Pharmaceutics 12:604. https://doi.org/10.3390/pharmaceutics12070604
Hou S, Wang F, Li Y, Li Y, Wang M, Sun D, Sun C (2012) Pharmacokinetic study of mangiferin in human plasma after oral administration. Food Chem 132:289–294. https://doi.org/10.1016/j.foodchem.2011.10.079
Ismail A, Nasr M, Sammour O (2020) Nanoemulsion as a feasible and biocompatible carrier for ocular delivery of travoprost: improved pharmacokinetic/pharmacodynamic properties. Int J Pharm 583:119402. https://doi.org/10.1016/j.ijpharm.2020.119402
Jafari S, Derakhshankhah H, Alaei L, Fattahi A, Varnamkhasti BS, Saboury AA (2019) Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed Pharmacother 109:1100–1111. https://doi.org/10.1016/j.biopha.2018.10.167
Jain PK, Kharya M, Gajbhiye A (2013) Pharmacological evaluation of mangiferin herbosomes for antioxidant and hepatoprotection potential against ethanol induced hepatic damage. Drug Dev Ind Pharm 39:1840–1850. https://doi.org/10.3109/03639045.2012.738685
Jamwal D, Saini P, Tomar PC, Ghosh A (2022) Perspectives on the potential of mangiferin as a nutraceutical: a review. Food Sci Nutr. https://doi.org/10.1108/NFS-01-2022-0013
Jyotshna, Khare P, Shanker K (2016) Mangiferin: a review of sources and interventions for biological activities. BioFactors 42:504–514. https://doi.org/10.1002/biof.1308
Kammala K (2015) Structural elucidation of possible metabolic profile of mangiferin by oral and intraperitoneal administration. J Pharm Drug Deliv Res 4:1000128. https://doi.org/10.4172/2325-9604.1000128
Khurana RK, Rao S, Beg S, Katare OP, Singh B (2016a) Systematic development and validation of a thin-Layer densitometric bioanalytical method for estimation of mangiferin employing analytical quality by design (AQbD) approach. J Chromatogr Sci 54:829–841. https://doi.org/10.1093/chromsci/bmw001
Khurana RK, Kaur R, Lohan S, Singh KK, Singh B (2016b) Mangiferin: a promising anticancer nioactive. Pharm Pat Anal 5:169–181. https://doi.org/10.4155/ppa-2016-0003
Khurana RK, Kaur R, Kaur M, Kaur R, Kaur J, Kaur H, Singh B (2017a) Exploring and validating physicochemical properties of mangiferin through GastroPlus® software. Future Sci OA 3:FSO167. https://doi.org/10.4155/fsoa-2016-0055
Khurana RK, Bansal AK, Beg S, Burrow AJ, Katare OP, Singh KK, Singh B (2017b) Enhancing biopharmaceutical attributes of phospholipid complex-loaded nanostructured lipidic carriers of mangiferin: systematic development, characterization and evaluation. Int J Pharm 518:289–306. https://doi.org/10.1016/j.ijpharm.2016.12.044
Khurana RK, Gaspar BL, Welsby G, Katare OP, Singh KK, Singh B (2018) Improving the biopharmaceutical attributes of mangiferin using vitamin E-TPGS co-loaded self-assembled phosholipidic nano-mixed micellar systems. Drug Deliv Transl Res 8:617–632. https://doi.org/10.1007/s13346-018-0498-4
Kianfar E (2021) Protein nanoparticles in drug delivery: animal protein, plant proteins and protein cages, albumin nanoparticles. J Nanobiotechnology 19:159. https://doi.org/10.1186/s12951-021-00896-3
Kumar A, Zhang X, Liang XJ (2013) Gold nanoparticles: emerging paradigm for targeted drug delivery system. Biotechnol 31:593–606. https://doi.org/10.1016/j.biotechadv.2012.10.002
Leiro J, Arranz JA, Yanez M, Ubeira FM, Sanmartin ML, Orallo F (2004) Expression profiles of genes involved in the mouse nuclear factor-kappa B signal transduction pathway are modulated by mangiferin. Int Immunopharmacol 4:763–778. https://doi.org/10.1016/j.intimp.2004.03.002
Lemus-Molina Y, Sanchez-Gomez MV, Gado-Hernandez R, Matute C (2009) Mangifera indica L. extract attenuates glutamateinduced neurotoxicity on rat cortical neurons. Neurotoxicology 30:1053–1058. https://doi.org/10.1016/j.neuro.2009.06.012
Li H, Wang Q, Ding Y, Bao C, Li W (2017) Mangiferin ameliorates Porphyromonas gingivalis induced experimental periodontitis by inhibiting phosphorylation of nuclear factor -κB and Janus kinase 1–signal transducer and activator of transcription signaling pathways. J Periodontal Res 52:1–7. https://doi.org/10.1111/jre.12360
Liang J, Li F, Lin J, Song S, Liao X, Gao C, Yang B (2019) Host-guest inclusion systems of mangiferin and polyamine-b-cyclodextrins: preparation, characterization and anti-cancer activity. J Mol Struct 1193:207–214. https://doi.org/10.1016/j.molstruc.2019.05.015
Liu R, Liu Z, Zhang C, Zhang B (2012) Nanostructured lipid carriers as novel ophthalmic delivery system for mangiferin: improving in vivo ocular bioavailability. J Pharm Sci 101:3833–3844. https://doi.org/10.1002/jps.23251
Louisa M, Soediro TM, Suyatna FD (2014) In vitro modulation of P-glycoprotein, MRP-1 and BCRP expression by mangiferin in doxorubicin-treated MCF-7 cells. Asian Pac J Cancer Prev 15:1639–1642. https://doi.org/10.7314/apjcp.2014.15.4.1639
Ma H, Chen H, Sun L, Tong L, Zhang T (2014) Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia 93:54–61. https://doi.org/10.1016/j.fitote.2013.10.016
Mei S, Perumal M, Battino M, Kitts DD, Xiao J, Ma H, Chen X (2021a) Mangiferin: a review of dietary sources, absorption, metabolism, bioavailability, and safety. Crit Rev Food Sci Nutr 149:111997–111919. https://doi.org/10.1080/10408398.2021.1983767
Mei S, Ma H, Chen X (2021b) Anticancer and anti-inflammatory properties of mangiferin: a review of its molecular mechanisms. Food Chem Toxicol 149:111997. https://doi.org/10.1016/j.fct.2021.111997
Mellaerts R, Roeffaers MBJ, Houthoofd K, Van Speybroeck M, De Cremer G, Jammaer JAG, Van den Mooter G, Augustijns P, Hofkens J, Martens JA (2011) Molecular organization of hydrophobic molecules and co-adsorbed water in SBA-15 ordered mesoporous silica material. Phys Chem Chem Phys 13:2706–2713. https://doi.org/10.1039/C0CP01640C
Mittal S, Iqubal MK, Iqbal B, Gupta MM, Ali J, Baboota S (2020) A pervasive scientific overview on mangiferin in the prevention and treatment of various diseases with preclinical and clinical updates. J Complement Integr Med 18:9–21. https://doi.org/10.1515/jcim-2019-0250
Mohammad W, Aqil M, Goswami P, Agnihotri J, Nadeem S (2017) Nanoemulsion-based transdermal drug delivery system for the treatment of tuberculosis. Recent Pat Anti-Infect Drug Discov 12:107–119. https://doi.org/10.2174/1574891X12666170602075733
Moura JU, Barbosa GM, Genro C, Hernandez RD, Izquierdo SS, Gomes P, Fagan SB, Raffin RP (2014) Mangiferin-loaded polymeric nanocapsules. J Nanopharm Drug Deliv 2:87–92. https://doi.org/10.1166/jnd.2014.1040
Muller RH, Radtke M, Wissing SA (2002) Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev 54:131–155. https://doi.org/10.1016/s0169-409x(02)00118-7
Muruganandan S, Srinivasan K, Gupta S, Gupta PK, Lal J (2005) Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J Ethnopharmacol 97:497–501. https://doi.org/10.1016/j.jep.2004.12.010
Naggar VF, El-Khawas M, Ismail FA, Boraie NA (1992) Pectin, a possible matrix for oral sustained release preparations of water-soluble drugs. STP J Pharm Sci 2:227–234
Naseema A, Kovooru L, Behera AK, Kumar KPP, Srivastava P (2021) A critical review of synthesis procedures, applications and future potential of nanoemulsions. Adv Colloid Interface Sci 287:102318. https://doi.org/10.1016/j.cis.2020.102318
Nasr M, Abdel-Hamid S (2015) Lipid based nanocapsules: a multitude of biomedical applications. Curr Pharm Biotechnol 16:322–332. https://doi.org/10.2174/138920101604150218103555
Nasr M, Wahdan SA (2019) Neuroprotective effects of novel nanosystems simultaneously loaded with vinpocetine and piracetam after intranasal administration. Life Sci 226:117–129. https://doi.org/10.1016/j.lfs.2019.04.014
Nasr M, Awad GAS, Mansour S, Al Shamy A, Mortada ND (2011) A reliable predictive factorial model for entrapment optimization of a sodium bisphosphonate into biodegrabale microspheres. J Pharm Sci 110:612–621. https://doi.org/10.1002/jps.22297
Nikam AP, Ratnaparkhiand MP, Chaudhari SP (2014) Nanoparticles – an overview. Int J Res Dev Pharm L Sci 3:1121–1127
Nunez Selles AJ, Daglia M, Rastrelli L (2016) The potential role of mangiferin in cancer treatment through its immunomodulatory, anti-angiogenic, apoptopic, and gene regulatory effects. Biofactors 42:475–491. https://doi.org/10.1002/biof.1299
Ochocka R, Hering A, Stefanowicz-Hajduk J, Cal K, Barańska H (2017) The effect of mangiferin on skin: penetration, permeation and inhibition of ECM enzymes. PLoS ONE 12:e0181542. https://doi.org/10.1371/journal.pone.0181542
Ojewole JA (2005) Antiinflammatory, analgesic and hypoglycemic effects of Mangifera indica Linn. (Anacardiaceae) stem-bark aqueous extract. Methods Find Clin Exp Pharmacol Physiol 27:547–554. https://doi.org/10.1358/mf.2005.27.8.928308
Opatha SAT, Titapiwatanakun V, Chutoprapat R (2020) Transfersomes: a promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics 12:855. https://doi.org/10.3390/pharmaceutics12090855
Pal PB, Sinha K, Sil PC (2014) Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFα related and mitochondrial dependent apoptotic pathways in streptozotocin induced diabetic rats. PLoS ONE 9:e115364. https://doi.org/10.1371/journal.pone.0107220
Pan C, Pan Z, Hu J, Chen W, Zhou G, Lin W, Jin L, Xu C (2016) Mangiferin alleviates lipopolysaccharide and D-galactosamine-induced acute liver injury by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur J Pharmacol 770:85–91. https://doi.org/10.1016/j.ejphar.2015.12.006
Pardo-Andreu GL, Maurmann N, Reolon GR, Farias CB, Schwartsmann G, Delgado-Hernandez R, Roesler R (2010) Mangiferin, a naturally occurring glucoxilxanthone improves long-term object recognition memory in rats. Eur J Pharmacol 635:124–128. https://doi.org/10.1016/j.ejphar.2010.03.011
Peng ZG, Yao YB, Yang J, Tang YL, Huang X (2015) Mangiferin induces cell cycle arrest at G2/M phase through ATR-Chk1 pathway in HL-60 leukemia cells. Genet Mol Res 14:4989–5002. https://doi.org/10.4238/2015.May.12.2
Perez AP, Altube MJ, Schilrreff P, Apezteguia G, Celes F, Santana Z, Susana I, Camila O, Eder R, Lilia M, Maria J (2016) Topical amphotericin B in ultradeformable liposomes: formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surf B 139:190–198. https://doi.org/10.1016/j.colsurfb.2015.12.003
Pleguezuelos-Villaa M, Náchera A, Hernándezc MJ, Busod MAOV, Saurie AR, Díez-Salesa O (2019) Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. Int J Pharm 564:299–307. https://doi.org/10.1016/j.ijpharm.2019.04.056
Pleguezuelos-Villaa M, Diez-Salesa O, Mancac ML, Manconic M, Saurid AR, Escribano-Ferrere E, Náchera A (2020) Mangiferin glycethosomes as a new potential adjuvant for the treatment of psoriasis. Int J Pharm 573:118844. https://doi.org/10.1016/j.ijpharm.2019.118844
Porwa M, Rastogi V, Kumar A (2017) An overview on carbon nanotubes. MOJ Bioequiv Availab 3:114–116. https://doi.org/10.15406/mojbb.2017.03.00045
Prabhu S, Narayan S, Devi CS (2009) Mechanism of protective action of mangiferin on suppression of inflammatory response and lysosomal instability in rat model of myocardial infarction. Phytother Res 23:756–760. https://doi.org/10.1002/ptr.2549
Ramakrishna GA, Manohar SD, Bhanudas SR (2014) Ethosomes: carrier for enhanced transdermal drug delivery system. J Adv Pharm Res 4:380–387
Razura-Carmona FF, Pérez-Larios A, González-Silva N, Herrera-Martínez M, Medina-Torres L, Sáyago-Ayerdi SG, Sánchez Burgos JA (2019) Mangiferin-loaded polymeric nanoparticles: optical characterization, effect of antitopoisomerase I, and cytotoxicity. Cancers 11:1965. https://doi.org/10.3390/cancers11121965
Reddeman R, Glávits R, Endres JR, Clewell AE, Hirka G, Vértesi A, Béres E, Szakonyiné IP (2019) A toxicological evaluation of mango leaf extract (Mangifera indica) containing 60% mangiferin. J Toxicol 2019:4763015. https://doi.org/10.1155/2019/4763015
Rodeiro I, Hernandez S, Morffi J, Herrera JA, Gómez-Lechón MJ, Delgado R, Espinosa-Aguirre JJ (2012) Evaluation of genotoxicity and DNA protective effects of mangiferin, a glucosylxanthone isolated from Mangifera indica L. stem bark extract. Food Chem Toxicol 50:3360–3366. https://doi.org/10.1016/j.fct.2012.06.032
Saha S, Sadhukhan P, Sil PC (2016) Mangiferin: a xanthonoid with multipotent anti-inflammatory potential. Biofactors 10:459–474. https://doi.org/10.1002/biof.1292
Samadarsi R, Dutta D (2019) Design and characterization of mangiferin nanoparticles for oral delivery. J Food Eng 247:80–94. https://doi.org/10.1016/j.jfoodeng.2018.11.020
Samadarsi R, Dutta D (2020) Anti-oxidative effect of mangiferin-chitosan nanoparticles on oxidative stress-induced renal cells. Int J Biol Macromol 151:36–46. https://doi.org/10.1016/j.ijbiomac.2020.02.112
Samadarsi R, Mishra D, Dutt D (2020) Mangiferin nanoparticles fortified dairy beverage as a low glycemic food product: its quality attributes and antioxidant properties. Int J Food Sci Technol 55:589–600. https://doi.org/10.1111/ijfs.14310
Santonocito D, Vivero-Lopez M, Lauro MR, Torrisi C, Castelli F, Sarpietro MG, Puglia C (2022) Design of nanotechnological carriers for ocular delivery of mangiferin: preformulation study. Molecules 27:1328. https://doi.org/10.3390/molecules27041328
Sguizzato M, Ferrara F, Hallan SS, Baldisserotto A, Drechsler M, Malatesta M, Costanzo M, Cortesi R, Puglia C, Valacchi G, Esposito E (2021) Ethosomes and transethosomes for mangiferin transdermal delivery. Antioxidants 10:768. https://doi.org/10.3390/antiox10050768
Shi W, Deng J, Tong R, Yang Y, He X, Lv J, Wang H, Deng S, Qi P, Zhang D, Wang Y (2016) Molecular mechanisms underlying mangiferin-induced apoptosis and cell cycle arrest in A54 human lung carcinoma cells. Mol Med Rep 13:3423–3432. https://doi.org/10.3892/mmr.2016.4947
Shrestha H, Bala R, Arora S (2014) Lipid-based drug delivery systems. J Pharm 2014:801820. https://doi.org/10.1155/2014/801820
Silva CRP, Ferreira FD, Webler GD, Da Silva AOS, De Abreu FC, Fonseca EJS (2017) Encapsulation of mangiferin in ordered mesoporous silica type SBA-15: synthesis and characterization. Mater Res Express 4:065402. https://doi.org/10.1088/2053-1591/aa71e6
Tapeinosa C, Battaglini M, Ciofani G (2017) Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J Control Release 264:306–332. https://doi.org/10.1016/j.jconrel.2017.08.033
Telang M, Dhulap S, Mandhare A, Hirwani R (2013) Therapeutic and cosmetic applications of mangiferin: a patent review. Expert Opin Ther Pat 23:1561–1580. https://doi.org/10.1517/13543776.2013.836182
Telange DR, Sohail NK, Hemke AT, Kharkar PS, Pethe AM (2020) Phospholipid complex-loaded self-assembled phytosomal soft nanoparticles: evidence of enhanced solubility, dissolution rate, ex vivo permeability, oral bioavailability, and antioxidant potential of mangiferin. Drug Deliv Transl Res 11:1056–1083. https://doi.org/10.1007/s13346-020-00822-4
Thanitwatthanasak S, Sagis LMC, Chitprasert P (2019) Pluronic F127/Pluronic P123/vitamin E TPGS mixed micelles for oral delivery of mangiferin and quercetin: mixture-design optimization, micellization, and solubilization behavior. J Mol Liq 274:223–238. https://doi.org/10.1016/j.molliq.2018.10.089
Tian X, Xu Z, Li Z, Ma Y, Lian S, Guo X, Hu P, Gao Y, Huang C (2016a) Pharmacokinetics of mangiferin and its metabolite-norathyriol. Part 2: Influence of UGT, CYP450, P-gp, and enterobacteria and the potential interaction in Rhizoma Anemarrhenae decoction with timosaponin B2 as the major contributor. Biofactors 42:545–555. https://doi.org/10.1002/biof.1290
Tian X, Gao Y, Xu Z, Lian S, Ma Y, Guo X, Hu P, Li Z, Huang C (2016b) Pharmacokinetics of mangiferin and its metabolite-norathyriol. Part 1: Systemic evaluation of hepatic first-pass effect in vitro and in vivo. Biofactors 42:533–544. https://doi.org/10.1002/biof.1291
Vines JB, Yoon JH, Ryu NE, Lim DJ, Park H (2019) Gold nanoparticles for photothermal cancer therapy. Front Chem 7:167. https://doi.org/10.3389/fchem.2019.00167
Wang RR, Gao YD, Ma CH, Zhang XJ, Huang CG, Huang JF, Zheng YT (2011) Mangiferin, an anti-HIV-1 agent targeting protease and effective against resistant rtrains. Molecules 16:4264–4277. https://doi.org/10.3390/molecules16054264
Wen H, Jung H, Li X (2015) Drug delivery approaches in addressing clinical pharmacology-related issues: opportunities and challenges. AAPS J 17:1327–1340. https://doi.org/10.1208/s12248-015-9814-9
Wisutthathum S, Kamkaew N, Inchan A, Chatturong U, Paracha TU, Ingkaninan K, Wongwad E, Chootip K (2019) Extract of Aquilaria crassna leaves and mangiferin are vasodilators while showing no cytotoxicity. J Tradit Complement Med 9:237–242. https://doi.org/10.1016/j.jtcme.2018.09.002
Xiao J, Liu L, Zhong Z, Xiao C, Zhang J (2015) Mangiferin regulates proliferation and apoptosis in glioma cells by induction of microRNA-15b and inhibition of MMP-9 expression. Oncol Rep 33:2815–2820. https://doi.org/10.3892/or.2015.3919
Xiao W, Hou J, Ma J, Yu B, Ren J, Jin W, Wu J, Zheng D, Fan K (2021) Mangiferin loaded magnetic PCEC microspheres: preparation, characterization and antitumor activity studies in vitro. Arch Pharm Res 44:1–7. https://doi.org/10.1007/s12272-014-0485-3
Xing Y, Li R, Xue L, Chen M, Lu X, Duan Z, Zhou W, Li J (2022) Double emulsion (W/O/W) gel stabilised by polyglycerol polyricinoleate and calcium caseinate as mangiferin carrier: insights on formulation and stability properties. Int J Food Sci 57:5268–5279. https://doi.org/10.1111/ijfs.15856
Xuan X, Wang Y, Tian H, Pi J, Sun S, Zhang W (2012) Study on prescription of self-microemulsifying drug delivery system of mangiferin phospholipid complex. J Chin Med Mater 35:1508–1511
Yusri PZS, Ghazali NF, Mazlan NA, Lum PT, Noor AAM, Mani S, Sekar M (2020) Synthesis and characterization of mangiferin loaded N, O-CMC nanoparticles and its cytotoxic effect on osteosarcoma MG-63 cells. Res Pharm Sci 11:2136–2145. https://doi.org/10.26452/ijrps.v11i2.2162
Zhang Y, Liang R, Liu C, Yang C (2022) Improved stability and skin penetration through glycethosomes loaded with glycyrrhetinic acid. Int J Cosmet Sci 44:249–261. https://doi.org/10.1111/ics.12771
Zhao D, Huo Q, Feng J, Chmelka BF, Stucky GD (1998) Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J Am Chem 120:6024–6036. https://doi.org/10.1021/ja974025i
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MN, SB, and NM designed the study. SA collected data and wrote the initial draft of the manuscript. RFA completed the main content of the manuscript. SB, MN, RFA, and NM participated in revising the manuscript. All authors have read the final manuscript and approved its submission.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Supplementary information
ESM 1
(PDF 281 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barakat, S., Nasr, M., Ahmed, R.F. et al. Recent Formulation Advances of Mangiferin. Rev. Bras. Farmacogn. 32, 871–882 (2022). https://doi.org/10.1007/s43450-022-00297-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-022-00297-z