Abstract
Background
The present study examined whether inhibition of guanylate cyclase (GC) is associated with the plasticity-related microtubule-stabilizing protein tau phosphorylation in the dentate gyrus (DG) of hippocampal formation.
Methods
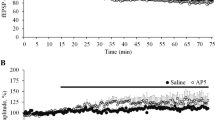
To address this issue, methylene blue (MB 50 μM) or saline was infused into the DG starting from the induction of long-term potentiation (LTP) or depression (LTD) for 1 h. Then, protein phosphatase 1 alpha (PP1α), glycogen synthase kinase 3 beta (GSK3β), and tau total and phosphorylated protein levels were measured in these hippocampi using western blotting. LTP and LTD were induced by application of high- and low-frequency stimulation protocols (HFS and LFS), respectively. 5-min averages of the excitatory postsynaptic potential (EPSP) slopes and population spike amplitudes at the end of recording were averaged to measure the magnitude of LTP or LTD.
Results
Low-frequency stimulation protocols was unable to phosphorylate thr181 and thr231epitopes of tau, but possessed kinase activity similar to the HFS in phosphorylation of ser396 and ser416 epitopes. MB infusion during LTD induction attenuated LTD, prevented EPSP/spike dissociation and increased tau phosphorylation at ser396 and ser416 epitopes, without changing tau phosphorylation at thr181 and thr231 epitopes. Neither LTP nor LTP-related tau phosphorylation state was changed by MB infusion.
Conclusion
Although MB can benefit to stabilize the balance between LTP and LTD, and to fix the increased spike wave discharges, it might trigger deregulation of tau phosphorylation, leading to the development of Alzheimer’s disease by a mechanism that goes awry during induction of LTD. Thereby detailed studies to reveal more precise evidence for the use of MB in this disease are needed.





Similar content being viewed by others
References
Abraham WC, Jones OD, Glanzman DL. Is plasticity of synapses the mechanism of long-term memory storage? Npj Sci Learn. 2019;4:1–10.
Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacol. 2008;33:18–41.
Lee H-K, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–9.
Kimura T, Whitcomb DJ, Jo J, Regan P, Piers T, Heo S, et al. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos T R Soc B. 2014;369:1–11.
Ittner A, Chua SW, Bertz J, Volkerling A, van der Hoven J, Gladbach A, et al. Site-specific phosphorylation of tau inhibits amyloid-beta toxicity in Alzheimer’s mice. Science. 2016;354:904–8.
Chen Q, Zhou Z, Zhang L, Wang Y, Zhang YW, Zhong M, et al. Tau protein is involved in morphological plasticity in hippocampal neurons in response to BDNF. Neurochem Int. 2012;60:233–42.
Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, et al. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci. 2011;31:700–11.
Shipton OA, Leitz JR, Dworzak J, Acton CEJ, Tunbridge EM, Denk F, et al. Tau protein is required for amyloid beta-induced impairment of hippocampal long-term potentiation. J Neurosci. 2011;31:1688–92.
Regan P, Piers T, Yi JH, Kim DH, Huh S, Park SJ, et al. Tau phosphorylation at serine 396 residue is required for hippocampal LTD. J Neurosci. 2015;35:4804–12.
Babür E, Tan B, Delibaş S, Yousef M, Dursun N, Süer C. Depotentiation of long-term potentiation is associated with epitope-specific tau hyper-/hypophosphorylation in the hippocampus of adult rats. J Mol Neurosci. 2018;67:193–203.
Kobayashi S, Tanaka T, Soeda Y, Almeida OFX, Takashima A. Local somatodendritic translation and hyperphosphorylation of tau protein triggered by AMPA and NMDA receptor stimulation. EBioMedicine. 2017;20:120–6.
Frandemiche ML, De Seranno S, Rush T, Borel E, Elie A, Arnal I, et al. Activity-dependent tau protein translocation to excitatory synapse is disrupted by exposure to amyloid-beta oligomers. J Neurosci. 2014;34:6084–97.
Braak E, Braak H, Mandelkow EM. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. 1994;87:554–67.
Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016;19:1085–92.
Gotz J, Probst A, Spillantini MG, Schafer T, Jakes R, Burki K, et al. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. Embo J. 1995;14:1304–13.
Wang X, Robinson PJ. Cyclic GMP-dependent protein kinase and cellular signaling in the nervous system. J Neurochem. 1997;68:443–56.
Thompson SH. Cyclic GMP-gated channels in a sympathetic neuron cell line. J Gen Physiol. 1997;110:155–64.
Haby C, Lisovoski F, Aunis D, Zwiller J. Stimulation of the cyclic-Gmp pathway by no induces expression of the immediate-early genes C-Fos and Junb in Pc12 cells. J Neurochem. 1994;62:496–501.
Chalimoniuk M, Strosznajder JB. Aging modulates nitric oxide synthesis and cGMP levels in hippocampus and cerebellum—effects of amyloid beta peptide. Mol Chem Neuropathol. 1998;35:77–95.
Schirmer RH, Adler H, Pickhardt M, Mandelkow E. Lest we forget you—methylene blue. Neurobiol Aging. 2011;32(2325):e7–16.
Dormoi J, Pascual A, Briolant S, Amalvict R, Charras S, Baret E, et al. Proveblue (methylene blue) as an antimalarial agent: ın vitro synergy with dihydroartemisinin and atorvastatin. Antimicrob Agents Ch. 2012;56:3467–9.
Kwok ES, Howes D. Use of methylene blue in sepsis: a systematic review. J Intensive Care Med. 2006;21:359–63.
Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci USA. 1996;93:11213–8.
Congdon EE, Wu JW, Myeku N, Figueroa YH, Herman M, Marinec PS, et al. Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy. 2012;8:609–22.
Stack C, Jainuddin S, Elipenahli C, Gerges M, Starkova N, Starkov AA, et al. Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity. Hum Mol Genet. 2014;23:3716–32.
Wen Y, Li WJ, Poteet EC, Xie LK, Tan C, Yan LJ, et al. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem. 2011;286:16504–15.
Arai T, Hasegawa M, Nonoka T, Kametani F, Yamashita M, Hosokawa M, et al. Phosphorylated and cleaved TDP-43 in ALS, FTLD and other neurodegenerative disorders and in cellular models of TDP-43 proteinopathy. Neuropathology. 2010;30:170–81.
Sontag EM, Lotz GP, Agrawal N, Tran A, Aron R, Yang G, et al. Methylene blue modulates huntingtin aggregation intermediates and is protective in Huntington’s disease models. J Neurosci. 2012;32:11109–19.
Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochem. 1999;38:3549–58.
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–26.
Sperber BR, Leight S, Goedert M, Lee VMY. Glycogen-synthase kinase-3-beta phosphorylates tau-protein at multiple sites in intact-cells. Neurosci Lett. 1995;197:149–53.
Li T, Paudel HK. Glycogen synthase kinase 3beta phosphorylates Alzheimer’s disease-specific Ser396 of microtubule-associated protein tau by a sequential mechanism. Biochem. 2006;45:3125–33.
Yagishita S, Murayama M, Ebihara T, Maruyama K, Takashima A. Glycogen synthase kinase 3 beta-mediated phosphorylation in the most c-terminal region of protein interacting with C kinase 1 (PICK1) regulates the binding of PICK1 to glutamate receptor subunit GluA2. J Biol Chem. 2015;290:29438–48.
Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–17.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Acad; 1998.
Suer C, Dolu N, Artis AS, Sahin L, Aydogan S. Electrophysiological evidence of biphasic action of carnosine on long-term potentiation in urethane-anesthetized rats. Neuropeptides. 2011;45:77–81.
Artis AS, Bitiktas S, Taskin E, Dolu N, Liman N, Suer C. Experimental hypothyroidism delays field excitatory post-synaptic potentials and disrupts hippocampal long-term potentiation in the dentate gyrus of hippocampal formation and Y-maze performance in adult rats. J Neuroendocrinol. 2012;24:422–33.
Castellani GC, Quinlan EM, Bersani F, Cooper LN, Shouval HZ. A model of bidirectional synaptic plasticity: from signaling network to channel conductance. Learn Memory. 2005;12:423–32.
Ginimuge PR, Jyothi S. Methylene blue: revisited. J Anaesthesiol Clin Pharmacol. 2010;26:517.
Hopper RA, Garthwaite J. Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J Neurosci. 2006;26:11513–21.
Lu YF, Kandel ER, Hawkins RD. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci. 1999;19:10250–61.
Kleppisch T, Pfeifer A, Klatt P, Ruth P, Montkowski A, Fassler R, et al. Long-term potentiation in the hippocampal CA1 region of mice lacking cGMP-dependent kinases is normal and susceptible to inhibition of nitric oxide synthase. J Neurosci. 1999;19:48–55.
Wu J, Wang Y, Rowan MJ, Anwyl R. Evidence for involvement of the cGMP–protein kinase G signaling system in the induction of long-term depression, but not long-term potentiation, in the dentate gyrus in vitro. J Neurosci. 1998;18:3589–96.
Vutskits L, Briner A, Klauser P, Gascon E, Dayer AG, Kiss JZ, et al. Adverse effects of methylene blue on the central nervous system. Anesthesiology. 2008;108:684–92.
Bradley J, Zhang YN, Bakin R, Lester HA, Ronnett GV, Zinn K. Functional expression of the heteromeric “olfactory” cyclic nucleotide-gated channel in the hippocampus: a potential effector of synaptic plasticity in brain neurons. J Neurosci. 1997;17:1993–2005.
Kramer RH, Molokanova E. Modulation of cyclic-nucleotide-gated channels and regulation of vertebrate phototransduction. J Exp Biol. 2001;204:2921–31.
Pasmanter N, Iheanacho F, Hashmi MF. Biochemistry, cyclic GMP. Treasure Island: StatPearls Publishing; 2019.
Tomasulo RA, Levy WB, Steward O. LTP-associated EPSP/spike dissociation in the dentate gyrus: GABAergic and non-GABAergic components. Brain Res. 1991;561:27–34.
Lopez-Rojas J, Heine M, Kreutz MR. Plasticity of intrinsic excitability in mature granule cells of the dentate gyrus. Sci Rep. 2016;6:21615.
Malleret G, Alarcon JM, Martel G, Takizawa S, Vronskaya S, Yin D, et al. Bidirectional regulation of hippocampal long-term synaptic plasticity and its influence on opposing forms of memory. J Neurosci. 2010;30:3813–25.
Solmaz V, Aksoy D, Yurtogulları S, Semiz M, Aydemir E, Erbas O. The effects of methylene blue and tadalafil in pentylenetetrazole induced convulsion model. Gulhane Med J. 2016;58:286–90.
Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801.
Fitzjohn SM, Morton RA, Kuenzi F, Rosahl TW, Shearman M, Lewis H, et al. Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein. J Neurosci. 2001;21:4691–8.
Chen Z, Liu R, Yang S-H, Dillon GH, Huang R. Methylene blue inhibits GABAA receptors by interaction with GABA binding site. Neuropharmacology. 2017;119:100–10.
Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci. 2002;25:510–7.
Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, et al. The role of GSK-3 in synaptic plasticity. Br J Pharmacol. 2008;153(Suppl 1):S428–37.
Doyere V, Errington M, Laroche S, Bliss T. Low-frequency trains of paired stimuli induce long-term depression in area CA1 but not in dentate gyrus of the intact rat. Hippocampus. 1996;6:52–7.
Bollen M, Stalmans W. The structure, role, and regulation of type 1 protein phosphatases. Crit Rev Biochem Mol Biol. 1992;27:227–81.
Morishita W, Connor JH, Xia H, Quinlan EM, Shenolikar S, Malenka RC. Regulation of synaptic strength by protein phosphatase 1. Neuron. 2001;32:1133–48.
Combs B, Christensen K, Richards C, Kanaan NM. The Interaction between tau and protein phosphatase 1 is affected by p301L mutation. J Alzheimer’s Dis. 2018;14:P1136–7.
LaPointe NE, Morfini G, Pigino G, Gaisina IN, Kozikowski AP, Binder LI, et al. The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. J Neurosci Res. 2009;87:440–51.
Yamamoto H, Hiragami Y, Murayama M, Ishizuka K, Kawahara M, Takashima A. Phosphorylation of tau at serine 416 by Ca2+/calmodulin-dependent protein kinase II in neuronal soma in brain. J Neurochem. 2005;94:1438–47.
Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25:59–68.
Lei P, Ayton S, Bush AI, Adlard PA. GSK-3 in neurodegenerative diseases. Int J Alzheimers Dis. 2011;2011:189246.
Engel T, Goni-Oliver P, Lucas JJ, Avila J, Hernandez F. Chronic lithium administration to FTDP-17 tau and GSK-3beta overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. J Neurochem. 2006;99:1445–55.
Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–9.
Hernandez F, Langa E, Cuadros R, Avila J, Villanueva N. Regulation of GSK3 isoforms by phosphatases PP1 and PP2A. Mol Cell Biochem. 2010;344:211–5.
Hochgrafe K, Sydow A, Matenia D, Cadinu D, Konen S, Petrova O, et al. Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human Tau. Acta Neuropathol. 2015;3:25.
Taniguchi S, Suzuki N, Masuda M, Hisanaga S-i, Iwatsubo T, Goedert M, et al. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J Biol Chem. 2005;280:7614–23.
Howland RH. Methylene blue: the long and winding road from stain to brain: part 2. J Psychosoc Nurs Ment Health Serv. 2016;54:21–4.
O’Leary JC 3rd, Li Q, Marinec P, Blair LJ, Congdon EE, Johnson AG, et al. Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol neurodegener. 2010;5:45.
Wischik CM, Bentham P, Wischik DJ, Seng KM. Tau aggregation inhibitor (TAI) therapy with rember arrests disease progression in mild and moderate Alzheimer’s disease over 50 weeks. J Alzheimer’s Dis. 2008;4:T167.
Yamashita M, Nonaka T, Arai T, Kametani F, Buchman VL, Ninkina N, et al. Methylene blue and dimebon inhibit aggregation of TDP-43 in cellular models. FEBS Lett. 2009;583:2419–24.
Soeda Y, Saito M, Maeda S, Ishida K, Nakamura A, Kojima S, et al. Methylene blue ınhibits formation of tau fibrils but not of granular tau oligomers: a plausible key to understanding failure of a clinical trial for Alzheimer’s disease. J Alzheimer’s Dis. 2019;68(4):1677–86.
van Bebber F, Paquet D, Hruscha A, Schmid B, Haass C. Methylene blue fails to inhibit Tau and polyglutamine protein dependent toxicity in zebrafish. Neurobiol Dis. 2010;39:265–71.
Zhang YH, Wang DW, Xu SF, Zhang S, Fan YG, Yang YY, et al. alpha-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S tau transgenic mice. Redox Biol. 2018;14:535–48.
Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM. Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10:1089–99.
Acknowledgements
We would like to thank the Erciyes University Research Fund for their financial support under Project Number TYL-2018-8661.
Author information
Authors and Affiliations
Contributions
CS: wrote article, analyzing of electrophysiological experiments, feedback of manuscript. NY: electrophysiological experiments, western blot experiments. ÖB: electrophysiological experiments, western blot experiments. BT: western blot experiments, analyzing of western blot images, feedback of manuscript. ND: planning, initiator of project, sectioning, wrote article, feedback of manuscript. All the authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Süer, C., Yıldız, N., Barutçu, Ö. et al. Long-term depression-related tau phosphorylation is enhanced by methylene blue in healthy rat hippocampus. Pharmacol. Rep 73, 828–840 (2021). https://doi.org/10.1007/s43440-021-00254-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-021-00254-y