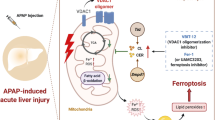
Abstract
Drug-induced liver injury (DILI) is a major cause of acute liver failure and drug withdrawal. Cytochrome P450 (CYP) 2E1 is involved in the metabolism of several drugs, and can induce liver injury through the production of toxic metabolites and the generation of reactive oxygen species. This study aimed to elucidate the role of Wnt/β-catenin signaling in CYP2E1 regulation for drug-induced hepatotoxicity. To achieve this, mice were administered cisplatin or acetaminophen (APAP) 1 h after treatment with the CYP2E1 inhibitor dimethyl sulfoxide (DMSO), and histopathological and serum biochemical analyses were performed. APAP treatment induced hepatotoxicity, as evidenced by an increase in liver weight and serum ALT levels. Moreover, histological analysis indicated severe injury, including apoptosis, in the liver tissue of APAP-treated mice, which was confirmed by TUNEL assay. Additionally, APAP treatment suppressed the antioxidant capacity of the mice and increased the expression of the DNA damage markers γ-H2AX and p53. However, these effects of APAP on hepatotoxicity were significantly attenuated by DMSO treatment. Furthermore, the activation of Wnt/β-catenin signaling using the Wnt agonist CHIR99021 (CHIR) increased CYP2E1 expression in rat liver epithelial cells (WB-F344), whereas treatment with the Wnt/β-catenin antagonist IWP-2 inhibited nuclear β-catenin and CYP2E1 expression. Interestingly, APAP-induced cytotoxicity in WB-F344 cells was exacerbated by CHIR treatment and suppressed by IWP-2 treatment. Overall, these results showed that the Wnt/β-catenin signaling is involved in DILI through the upregulation of CYP2E1 expression by directly binding the transcription factor β-cat/TCF to the Cyp2e1 promoter, thus exacerbating DILI.



Similar content being viewed by others
Data Availability
Data generated and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
Abbreviations
- ALT:
-
alanine aminotransferase
- ALF:
-
acute liver failure
- APAP:
-
acetaminophen
- AST:
-
aspartate aminotransferase
- ChIP:
-
chromatin immunoprecipitation
- CHIR:
-
CHIR99021
- CYP:
-
Cytochrome P450
- DILI:
-
Drug-Induced Liver Injury
- DMSO:
-
dimethyl sulfoxide
- GSH:
-
glutathione
- GPX:
-
glutathione peroxidase
- H&E:
-
hematoxylin and eosin
- HSCs:
-
hepatic stellate cells
- LEF:
-
lymphoid enhancer factor
- LRP-5/6:
-
lipoprotein receptor-related proteins 5 and 6
- NAPQI:
-
N-acetyl-p-benzoquinone imine
- PBST:
-
phosphate-buffered saline with 0.1% Tween ® 20 detergent Pt platinum
- ROS:
-
reactive oxygen species
- SOD1:
-
superoxide dismutase 1
- TBS:
-
tris-buffered saline
- TCF:
-
T-cell factor
- TUNEL:
-
terminal deoxynucleotidyl transferase dUTP nick end labeling
References
Suk KT, Baik SK, Yoon JH, Cheong JY, Paik YH, Lee CH, Kim YS, Lee JW, Kim DJ, Cho SW, Hwang SG, Sohn JH, Kim MY, Kim YB, Kim JG, Cho YK, Choi MS, Kim HJ, Lee HW, Kim SU, Kim JK, Choi JY, Jun DW, Tak WY, Lee BS, Jang BK, Chung WJ, Kim HS, Jang JY, Jeong SW, Kim SG, Kwon OS, Jung YK, Choe WH, Lee JS, Kim IH, Shim JJ, Cheon GJ, Bae SH, Seo YS, Choi DH, Jang SJ, Korean Association for the Study of the L (2012) Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol 18:1–21. https://doi.org/10.3350/kjhep.2012.18.1.1
Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, Possamai LA, Bruce M, McPhail M, Starling C (2012) Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology 56:735–746
Pandit A, Sachdeva T, Bafna P (2012) Drug-induced hepatotoxicity: a review. J Appl Pharm Sci 5:233–243
Metushi I, Uetrecht J, Phillips E (2016) Mechanism of isoniazid-induced hepatotoxicity: then and now. Brit J Clin Pharmaco 81:1030–1036. https://doi.org/10.1111/bcp.12885
Ramappa V, Aithal GP (2013) Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. J Clin Exp Hepatol 3:37–49. https://doi.org/10.1016/j.jceh.2012.12.001
Delord JP, Puozzo C, Lefresne F, Bugat R (2009) Combination chemotherapy of vinorelbine and cisplatin: a phase I pharmacokinetic study in patients with metastatic solid tumors. Anticancer Res 29:553–560
Go RS, Adjei AA (1999) Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol 17:409–422. https://doi.org/10.1200/JCO.1999.17.1.409
Chirino YI, Hernandez-Pando R, Pedraza-Chaverri J (2004) Peroxynitrite decomposition catalyst ameliorates renal damage and protein nitration in cisplatin-induced nephrotoxicity in rats. BMC Pharmacol 4:20. https://doi.org/10.1186/1471-2210-4-20
Quintanilha JCF, de Sousa VM, Visacri MB, Amaral LS, Santos RMM, Zambrano T, Salazar LA, Moriel P (2017) Involvement of cytochrome P450 in cisplatin treatment: implications for toxicity. Cancer Chemother Pharmacol 80:223–233. https://doi.org/10.1007/s00280-017-3358-x
Giridharan VV, Thandavarayan RA, Bhilwade HN, Ko KM, Watanabe K, Konishi T (2012) Schisandrin B, attenuates cisplatin-induced oxidative stress, genotoxicity and neurotoxicity through modulating NF-κB pathway in mice. Free Radic Res 46:50–60
Fuertes MA, Castilla J, Alonso C, Perez JM (2003) Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem 10:257–266. https://doi.org/10.2174/0929867033368484
Whitcomb DC (1994) Acetaminophen poisoning and liver function. N Engl J Med 331:1311–1312
Fontana RJ (2008) Acute liver failure including acetaminophen overdose. Med Clin North Am 92:761–794, viii. https://doi.org/10.1016/j.mcna.2008.03.005
Hinson JA, Roberts DW, James LP (2010) Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 369–405. https://doi.org/10.1007/978-3-642-00663-0_12
Fisher JE, McKenzie TJ, Lillegard JB, Yu Y, Juskewitch JE, Nedredal GI, Brunn GJ, Yi ES, Malhi H, Smyrk TC, Nyberg SL (2013) Role of Kupffer cells and toll-like receptor 4 in acetaminophen-induced acute liver failure. J Surg Res 180:147–155. https://doi.org/10.1016/j.jss.2012.11.051
Gonzalez FJ (1988) The molecular biology of cytochrome P450s. Pharmacol Rev 40:243–288
Yim SK, Kim K, Chun S, Oh T, Jung W, Jung K, Yun CH (2020) Screening of human CYP1A2 and CYP3A4 inhibitors from Seaweed in Silico and in Vitro. Mar Drugs 18:603. https://doi.org/10.3390/md18120603
Topletz AR, Dennison JB, Barbuch RJ, Hadden CE, Hall SD, Renbarger JL (2013) The relative contributions of CYP3A4 and CYP3A5 to the metabolism of vinorelbine. Drug Metab Dispos 41:1651–1661. https://doi.org/10.1124/dmd.113.051094
Towles JK, Clark RN, Wahlin MD, Uttamsingh V, Rettie AE, Jackson KD (2016) Cytochrome P450 3A4 and CYP3A5-Catalyzed bioactivation of Lapatinib. Drug Metab Dispos 44:1584–1597. https://doi.org/10.1124/dmd.116.070839
Lu Y, Cederbaum AI (2006) Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol Sci 89:515–523. https://doi.org/10.1093/toxsci/kfj031
Jaeschke H, McGill MR, Ramachandran A (2012) Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev 44:88–106. https://doi.org/10.3109/03602532.2011.602688
Gonzalez FJ (2001) The use of gene knockout mice to unravel the mechanisms of toxicity and chemical carcinogenesis. Toxicol Lett 120:199–208. https://doi.org/10.1016/s0378-4274(01)00296-x
Wang S, Sugamori KS, Tung A, McPherson JP, Grant DM (2015) N-hydroxylation of 4-aminobiphenyl by CYP2E1 produces oxidative stress in a mouse model of chemically induced liver cancer. Toxicol Sci 144:393–405. https://doi.org/10.1093/toxsci/kfv006
Zong H, Armoni M, Harel C, Karnieli E, Pessin JE (2012) Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. Am J Physiol Endocrinol Metab 302:E532–E539. https://doi.org/10.1152/ajpendo.00258.2011
Kong X, Guo D, Liu S, Zhu Y, Yu C (2021) Incidence, characteristics and risk factors for drug-induced liver injury in hospitalized patients: a matched case-control study. Brit J Clin Pharmaco 87:4304–4312. https://doi.org/10.1111/bcp.14847
Lee WM (2017) Acetaminophen (APAP) hepatotoxicity-Isn’t it time for APAP to go away? J Hepatol 67:1324–1331. https://doi.org/10.1016/j.jhep.2017.07.005
Tarantino G, Di Minno MND, Capone D (2009) Drug-induced liver injury: is it somehow foreseeable? World J gastroenterology: WJG 15:2817
Zanger UM, Schwab M (2013) Pharmacology & therapeutics cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138:103–141
Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, Monga SP (2014) beta-catenin signaling in murine liver zonation and regeneration: a wnt-wnt situation! Hepatology 60:964–976. https://doi.org/10.1002/hep.27082
Hailfinger S, Jaworski M, Braeuning A, Buchmann A, Schwarz M (2006) Zonal gene expression in murine liver: lessons from tumors. Hepatology 43:407–414. https://doi.org/10.1002/hep.21082
Thompson MD, Monga SP (2007) WNT/beta-catenin signaling in liver health and disease. Hepatology 45:1298–1305. https://doi.org/10.1002/hep.21651
Monga SP (2011) Role of Wnt/beta-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol 43:1021–1029. https://doi.org/10.1016/j.biocel.2009.09.001
Lehwald N, Tao GZ, Jang KY, Papandreou I, Liu B, Liu B, Pysz MA, Willmann JK, Knoefel WT, Denko NC, Sylvester KG (2012) beta-catenin regulates hepatic mitochondrial function and energy balance in mice. Gastroenterology 143:754–764. https://doi.org/10.1053/j.gastro.2012.05.048
Kim M, Yun J-W, Shin K, Cho Y, Yang M, Nam KT, Lim K-M (2017) Expression levels of GABA-A receptor subunit alpha 3, Gabra3 and lipoprotein lipase, Lpl are associated with the susceptibility to acetaminophen-induced hepatotoxicity. Biomolecules & Therapeutics 25:112
Burenheide A, Kunze T, Clement B (2008) Inhibitory effects on cytochrome p450 enzymes of pentamidine and its amidoxime pro-drug. Basic Clin Pharmacol Toxicol 103:61–65. https://doi.org/10.1111/j.1742-7843.2008.00236.x
Liu Y, Flynn TJ, Xia M, Wiesenfeld PL, Ferguson MS (2015) Evaluation of CYP3A4 inhibition and hepatotoxicity using DMSO-treated human hepatoma HuH-7 cells. Cell Biol Toxicol 31:221–230. https://doi.org/10.1007/s10565-015-9306-9
Kon K, Kim JS, Jaeschke H, Lemasters JJ (2004) Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40:1170–1179. https://doi.org/10.1002/hep.20437
Redon CE, Nakamura AJ, Martin OA, Parekh PR, Weyemi US, Bonner WM (2011) Recent developments in the use of γ-H2AX as a quantitative DNA double-strand break biomarker. Aging 3:168
Lee HJ, Wolosin JM, Chung SH (2017) Divergent effects of Wnt/beta-catenin signaling modifiers on the preservation of human limbal epithelial progenitors according to culture condition. Sci Rep 7:15241. https://doi.org/10.1038/s41598-017-15454-x
Larson AM (2007) Acetaminophen hepatotoxicity. Clin Liver Dis 11:525–548, vi. https://doi.org/10.1016/j.cld.2007.06.006
Lu Y, Cederbaum AI (2006) Enhancement by pyrazole of lipopolysaccharide-induced liver injury in mice: role of cytochrome P450 2E1 and 2A5. Hepatology 44:263–274. https://doi.org/10.1002/hep.21241
Zaher H, Buters JT, Ward JM, Bruno MK, Lucas AM, Stern ST, Cohen SD, Gonzalez FJ (1998) Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol 152:193–199. https://doi.org/10.1006/taap.1998.8501
Algfeley SG, Al-Rejaie SS, Nagi MN (2019) Can acetaminophen/dimethyl sulfoxide formulation prevent accidental and intentional acetaminophen hepatotoxicity? Drug Dev Res 80:475–480
Hartman JH, Miller GP, Meyer JN (2017) Toxicological implications of mitochondrial localization of CYP2E1. Toxicol Res (Camb) 6:273–289. https://doi.org/10.1039/C7TX00020K
Lagadic-Gossmann D, Lerche C, Rissel M, Joannard F, Galisteo M, Guillouzo A, Corcos L (2000) The induction of the human hepatic CYP2E1 gene by interleukin 4 is transcriptional and regulated by protein kinase C. Cell Biol Toxicol 16:221–233. https://doi.org/10.1023/a:1007625925095
Zordoky BN, El-Kadi AO (2009) Role of NF-kappaB in the regulation of cytochrome P450 enzymes. Curr Drug Metab 10:164–178. https://doi.org/10.2174/138920009787522151
Nusse R (2003) Wnts and hedgehogs: lipid-modified proteins and similarities in signaling mechanisms at the cell surface. Development 130:5297–5305. https://doi.org/10.1242/dev.00821
Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M (2006) Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology 43:817–825. https://doi.org/10.1002/hep.21131
Nelson WJ, Nusse R (2004) Convergence of wnt, ß-catenin, and cadherin pathways. Science 303:1483–1487
Sekine S, Gutierrez PJ, Lan BY, Feng S, Hebrok M (2007) Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology 45:361–368. https://doi.org/10.1002/hep.21523
Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SP (2009) Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol 175:1056–1065. https://doi.org/10.2353/ajpath.2009.080976
Ma Y, Lv X, He J, Liu T, Wen S, Wang L (2016) Wnt agonist stimulates liver regeneration after small-for-size liver transplantation in rats. Hepatol Res 46:E154–E164. https://doi.org/10.1111/hepr.12553
Guo Y, Xiao L, Sun L, Liu F (2012) Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol Res 61:337–346. https://doi.org/10.33549/physiolres.932289
Cheng JH, She H, Han YP, Wang J, Xiong S, Asahina K, Tsukamoto H (2008) Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol 294:G39–G49. https://doi.org/10.1152/ajpgi.00263.2007
Ge W-S, Wang Y-J, Wu J-X, Fan J-G, Chen Y-W, Zhu L (2014) β-catenin is overexpressed in hepatic fibrosis and blockage of Wnt/β-catenin signaling inhibits hepatic stellate cell activation. Mol Med Rep 9:2145–2151
Lopez-Terrada D, Gunaratne PH, Adesina AM, Pulliam J, Hoang DM, Nguyen Y, Mistretta TA, Margolin J, Finegold MJ (2009) Histologic subtypes of hepatoblastoma are characterized by differential canonical wnt and notch pathway activation in DLK + precursors. Hum Pathol 40:783–794. https://doi.org/10.1016/j.humpath.2008.07.022
Acknowledgements
We thank Seoul National University Hospital (Seoul, Korea) for providing WB-F344 cells.
Funding
This work was supported by the Basic Science Research Program (2021R1A2C1009563) through the National Research Foundation (NRF) of Korea, funded by the Ministry of Science and ICT (MSIT), Republic of Korea.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by YSS, DBH, DHW, SYK, and CK. Data analysis were performed by YSS, DBH, JWP, YJ, and JWY. The first draft of the manuscript was written by YSS and DBH. And, writing-review, editing and supervision were performed by JWY. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All animal experimental procedures were performed after approval by the Institutional Animal Care and Use Committee at The Catholic University of Korea.
Conflict of Interest
The authors declare that they have no competing financial interests with respect to this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shin, YS., Hwang, DB., Won, DH. et al. The Wnt/β-catenin signaling pathway plays a role in drug-induced liver injury by regulating cytochrome P450 2E1 expression. Toxicol Res. 39, 443–453 (2023). https://doi.org/10.1007/s43188-023-00180-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-023-00180-6