Abstract
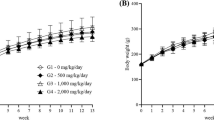
Quisqualis indica L. of Combretaceae family is a traditional medicine that is widely used for various gastrointestinal discomfort including stomach pain, constipation, and digestive problem. In this study, the potential repeated dose toxicity and genotoxicity of a standardized Quisqualis indica L. extract (HU033) were determined under good laboratory practice conditions. For the repeated dose toxicity test, HU033 was orally administered to Sprague–Dawley (SD) rats at doses of 500, 1000, and 2000 mg/kg/day for 13 consecutive weeks. The genotoxicity of HU033 was determined with a standard battery of genotoxicity test, including an in vitro bacterial reverse mutation test, an in vitro chromosomal aberration test, and an in vivo micronucleus test. After 13 weeks of repeated dose of HU033 by oral administration, there was no treatment related adverse clinical sign including food consumption, organ weights, and histopathological findings or significant decrement in bodyweight. The no-observed-adverse-effect level of HU033 was higher than 2000 mg/kg in both male and female SD rats. No target organs were identified. In addition, no evidence of HU033 genotoxicity was detected based on results from the bacterial reverse mutation test, chromosomal aberration test, and micronucleus test. Based on results of this study, HU033 could be safely used in food and medical products within the tested dose range.


Similar content being viewed by others
References
Rakotoarivelo NH, Rakotoarivony F, Ramarosandratana AV, Jeannoda VH, Kuhlman AR, Randrianasolo A, Bussmann RW (2015) Medicinal plants used to treat the most frequent diseases encountered in Ambalabe rural community, Eastern Madagascar. J Ethnobiol Ethnomed 11:68. https://doi.org/10.1186/s13002-015-0050-2
Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L (2011) Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med 8:1–10. https://doi.org/10.4314/ajtcam.v8i1.60483
Mosihuzzaman M (2012) Herbal medicine in healthcare-an overview. Nat Prod Commun 7:807–812. https://doi.org/10.1177/1934578x1200700628
Cohen PA, Ernst E (2010) Safety of herbal supplements: a guide for cardiologists. Cardiovasc Ther 28:246–253. https://doi.org/10.1111/j.1755-5922.2010.00193.x
Loya AM, González-Stuart A, Rivera JO (2009) Prevalence of polypharmacy, polyherbacy, nutritional supplement use and potential product interactions among older adults living on the United States-Mexico border: a descriptive, questionnaire-based study. Drugs Aging 26:423–436. https://doi.org/10.2165/00002512-200926050-00006
Qato DM, Alexander GC, Conti RM, Johnson M, Schumm R, Lindau ST (2008) Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 300:2867–2878. https://doi.org/10.1001/jama.2008.892
Kulshreshtha M, Srivastava G, Singh MP (2018) Pharmacognostical, anti-oxidant activity and high performance thin layer chromatography studies on leaves of Quisqualis indica Linn. Curr Tradit Med 4:53–67. https://doi.org/10.2174/2215083804666180118095645
Barik BS, Das S, Hussain T (2020) Pharmacognostic properties of Quisqualis indica Linn: against human pathogenic microorganisms: an insight review. Eur J Med Plants 31:87–103. https://doi.org/10.9734/ejmp/2020/v31i2030369
Kaiser MA, Islam MR, Rahman MS, Hossain MK, Rashid MA (2009) Total phenolic content, free radical scavenging activity and reducing power of Quisqualis indica Linn. Dhaka Univ J Pharm Sci 8:173–175. https://doi.org/10.3329/dujps.v8i2.6034
Singh VV, Jain J, Mishra AK (2017) Determination of antipyretic and antioxidant activity of Cassia occidentalis Linn methanolic seed extract. Pharmacogn J 9:913–916. https://doi.org/10.5530/pj.2017.6.143
Pal P, Singh A (2019) In-vitro antioxidant and anti-inflammatory activities of Quisqualis indica Linn. Leaves Extract J Adv Biol 21:1–13. https://doi.org/10.9734/jabb/2019/v21i130082
Gupta S, Singh A (2017) Antimicrobial, analgesic and anti-inflammatory activity reported on Tamarindus indica Linn root extract. Pharmacogn J. https://doi.org/10.5530/pj.2017.3.70
Wijerathne CU, Park HS, Jeong HY, Song JW, Moon OS, Seo YW, Won YS, Son HY, Lim JH, Yeon SH, Kwun HJ (2017) Quisqualis indica improves benign prostatic hyperplasia by regulating prostate cell proliferation and apoptosis. Bio Pharm Bull 40:2125–2133. https://doi.org/10.1248/bpb.b17-00468
Baek JM, Kim HJ, Nam MW, Park HJ, Yeon SH, Oh MH, Yoon JS, Kwon HJ, Lee KP, Lim JH (2019) Standardized seed extract of Quisqualis indica (HU033) attenuates testosterone propionate-induced benign prostatic hyperplasia via α1-adrenergic receptors and androgen/estrogen signaling. Prev Nutr Food Sci 24:492. https://doi.org/10.3746/pnf.2019.24.4.492
de Oliveira AM, do Nascimento MF, Ferreira MRA, de Moura DF, dos Santos Souza TG, da Silva GC, da Silva Ramo EH, Paiva PMG, de Medeiros PL, da Silva TG, Soares LAL (2016) Evaluation of acute toxicity, genotoxicity and inhibitory effect on acute inflammation of an ethanol extract of Morus alba L. (Moraceae) in mice. J Ethnopharmacol 194:162‒168. https://doi.org/10.1016/j.jep.2016.09.004
Furbee RB, Barlotta KS, Allen MK, Holstege CP (2006) Hepatotoxicity associated with herbal products. Clin Lab Med 26:227–241. https://doi.org/10.1016/j.cll.2006.02.005
Asif M (2012) A brief study of toxic effects of some medicinal herbs on kidney. Adv Biomed Res. https://doi.org/10.4103/2277-9175.100144
Kuete V, Tamokouu JDD (2014) Mutagenicity and carcinogenicity of African medicinal plants. In: Toxicological survey of African medicinal Plants, 1st edn. Elsevier, Netherlands, pp 277‒322
Organization for Economic Cooperation and Development (OECD) (1997) OECD guideline for testing of chemicals: bacterial reverse mutation test No. 471. OECD, Paris
OECD (1997) OECD guideline for testing of chemicals: in vitro mammalian chromosome aberration test No. 473. OECD, Paris
Ministry of Food and Drug Safety (MFDS) (2017) Guidelines for toxicity tests of pharmaceuticals. No. 2017-2071. MFDS, Sejong, Republic of Korea
OECD (1996) OECD guideline for testing of chemicals: mammalian erythrocyte micronucleus test No. 474. OECD, Paris
Jordan SA, Cunningham DG, Marles RJ (2010) Assessment of herbal medicinal products: challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol Appl Pharmacol 243:198–216. https://doi.org/10.1016/j.taap.2009
Liu C, Douglas RM (1998) Chinese herbal medicines in the treatment of acute respiratory infections: a review of randomized and controlled clinical trials. Med J Aust 169:579–582. https://doi.org/10.5694/j.1326-5377.1998.tb123423.x
Ansah C, Khan A, Gooderham NJ (2005) In vitro genotoxicity of the West African anti-malarial herbal Cryptolepis sanguinolenta and its major alkaloid cryptolepine. Toxicology 208:141–147. https://doi.org/10.1016/j.tox.2004.11.026
Zan MA, Ferraz A, Richter MF, Picada JN, de Andrade HH, Lehmann M, Dihl RR, Nunes E, Semedo J, Da Silva J (2013) In vivo genotoxicity evaluation of an artichoke (Cynara scolymus L.) aqueous extract. J Food Sci 78:367‒371. https://doi.org/10.1111/1750-3841.12034
Fateh AH, Mohamed Z, Chik Z, Alsalahi A, Zain SRM, Alshawsh MA (2019) Mutagenicity and genotoxicity effects of Verbena officinalis leaves extract in Sprague-Dawley rats. J Ethnopharmacol 235:88–99. https://doi.org/10.1016/j.jep.2019.02.007
Lin TC, Ma YT, Wu J, Hsu FL (1997) Tannins and related compounds from Quisqualis indica. J Chin Chem Soc 44:151–155. https://doi.org/10.1002/jccs.199700025
Ekawardhani S, Anggoro UT, Krissanti I (2021) Anthelmintic potential of medicinal plants against Ancylostoma caninum. Vet Med Int 2021:3879099. https://doi.org/10.1155/2021/3879099
Zhang W, Robert A, Vogensen SB, Howe JR (2006) The relationship between agonist potency and AMPA receptor kinetics. Biophys J 91:1336–1346. https://doi.org/10.1529/biophysj.106.084426
Kim DG, Kwon HJ, Lim JH, Kim JH, Lee KP (2020) Quisqualis indica extract ameliorates low urinary tract symptoms in testosterone propionate-induced benign prostatic hyperplasia rats. Lab Anim Res 36:1–10. https://doi.org/10.1186/s42826-020-00059-9
Thompson SL, Compton DA (2011) Chromosomes and cancer cells. Chromosome Res 19:433–444. https://doi.org/10.1007/s10577-010-9179-y
Hayashi M (2016) The micronucleus test—most widely used in vivo genotoxicity test. Genes Environ 38:1–6. https://doi.org/10.1186/s41021-016-0044-x
Funding
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the Agri-Bio Industry Technology Development Program funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (2016190262).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kim, JW., Kim, H., Park, H. et al. Repeated oral dose toxicity and genotoxicity of a standardized Quisqualis indica extract. Toxicol Res. 38, 577–589 (2022). https://doi.org/10.1007/s43188-022-00140-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-022-00140-6