Abstract
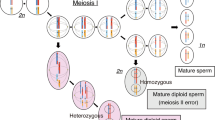
Androgenetic complete hydatidiform moles (CHMs) are associated with an increased risk of gestational trophoblastic neoplasia. P57KIP2 expression in hydatidiform moles is thought to be a powerful marker for differentiating CHMs from partial hydatidiform moles (PHMs). However, since there are so few such families clinically, very few studies have addressed the importance of p57KIP2-positive in the diagnosis and prognosis of CHM. This study aimed to emphasize the significance of the accurate diagnosis of rare CHM and careful follow-up. The classification of the hydatidiform mole was based on morphologic examination and p57KIP2 expression was determined by p57KIP2 immunohistochemical staining. Copy number variation sequencing was used to determine the genetic make-up of the mole tissues. In addition, the short tandem repeat polymorphism analysis was used to establish the parental origin of the moles. Finally, whole-exome sequencing was performed to identify the causal genetic variants associated with this case. In one Chinese family, the proband had numerous miscarriages throughout her two marriages. Morphologic evaluation and molecular genotyping accurately sub-classified two molar specimens as uniparental disomy CHM of androgenetic origin. Furthermore, p57KIP2 expression was found in cytotrophoblasts and villous stromal cells. In the tissue, there were hyperplasia trophoblastic cells and heteromorphic nuclei. In this family, no deleterious variant genes associated with recurrent CHM were detected. It is important to evaluate the prognostic value of p57KIP2 expression in androgenetic recurrent CHM. This knowledge may help to minimize erroneous diagnosis of CHMs as PHMs, as well as making us aware of the need to manage potential gestational trophoblastic neoplasia.




Similar content being viewed by others
References
Moein-Vaziri N, Fallahi J, Namavar-Jahromi B, et al. Clinical and genetic-epignetic aspects of recurrent hydatidiform mole: a review of literature. Taiwan J Obstet Gynecol. 2018;57:1–6.
Zhong T, Xie X, Zong T, et al. Lectin histochemical analysis of uterine natural killer cells in normal, hydatidiform molar and invasive molar pregnancy. Oncol Lett. 2018;16:6458–64.
Albright BB, Shorter JM, Mastroyannis SA, et al. Gestational trophoblastic neoplasia after human chorionic gonadotropin normalization following molar pregnancy: a systematic review and meta-analysis. Obstet Gynecol. 2020;135:12–23.
Giacometti C, Bellan E, Ambrosi A, et al. “While there is p57, there is hope.” The past and the present of diagnosis in first trimester abortions: diagnostic dilemmas and algorithmic approaches. A review. Placenta. 2021;25:S0143-4004(21)00060-6.
Chilosi M, Piazzola E, Lestani M, et al. Differential expression of p57kip2, a maternally imprinted cdk inhibitor, in normal human placenta and gestational trophoblastic disease. Lab Invest. 1998;78:269–76.
Fukunaga M. Immunohistochemical characterization of p57(KIP2) expression in early hydatidiform moles. Hum Pathol. 2002;33:1188–92.
Madi JM, Braga AR, Paganella MP, et al. Accuracy of p57KIP2 compared with genotyping for the diagnosis of complete hydatidiform mole: protocol for a systematic review and meta-analysis. Syst Rev. 2016;5:169.
Ronnett BM. Hydatidiform moles: ancillary techniques to refine diagnosis. Arch Pathol Lab Med. 2018;142:1485–502.
Nickkho-Amiry M, Horne G, Akhtar M, et al. Hydatidiform molar pregnancy following assisted reproduction. J Assist Reprod Genet. 2019;36:667–71.
Hui P, Buza N, Murphy KM, et al. Hydatidiform moles : genetic basis and precision diagnosis. Annu Rev Pathol. 2017;24:449–85.
Xing D, Adams E, Huang J, et al. Refined diagnosis of hydatidiform moles with p57 immunohistochemistry and molecular genotyping: updated analysis of a prospective series of 2217 cases. Mod Pathol. 2021;34:961–82.
Bynum J, Batista D, Xian R, et al. Tetraploid partial hydatidiform moles: molecular genotyping and determination of parental contributions. J Mol Diagn. 2020;22:90–100.
Tunster SJ, Van de Pette M, Creeth HDJ, et al. Fetal growth restriction in a genetic model of sporadic Beckwith-Wiedemann syndrome. Dis Model Mech. 2018;11:dmm035832.
Khawajkie Y, Mechtouf N, Nguyen NMP, et al. Comprehensive analysis of 204 sporadic hydatidiform moles: revisiting risk factors and their correlations with the molar genotypes. Mod Pathol. 2020;33:880–92.
Usui H, Sato A, Shozu M. Parental contribution to trisomy in heterozygous androgenetic complete moles. Sci Rep. 2020;10:17137.
Sunde L, Lund H, Sebire NJ, et al. Paternal hemizygosity in 11p15 in mole-like conceptuses: two case reports. Medicine (Baltimore). 2015;94:e1776.
Zaccaria S, Raphael BJ. Characterizing allele- and haplotype-specific copy numbers in single cells with CHISEL. Nat Biotechnol. 2021;39:207–14.
Usui H, Nakabayashi K, Maehara K, et al. Genome-wide single nucleotide polymorphism array analysis unveils the origin of heterozygous androgenetic complete moles. Sci Rep. 2019;9:12542.
Usui H, Sato A, Ota M, et al. Androgenetic complete hydatidiform moles with p57KIP2-positive immunostaining. Am J Clin Pathol. 2020;154:776–83.
Zheng XZ, Qin XY, Wang P, et al. Clinical application of STR genotyping diagnosis for hydatidiform mole and nonmolar gestation. Zhonghua Bing Li Xue Za Zhi. 2018;47:609–15.
Sebire NJ, May PC, Kaur B, et al. Abnormal villous morphology mimicking a hydatidiform mole associated with paternal trisomy of chromosomes 3,7,8 and unipaternal disomy of chromosome 11. Diagn Pathol. 2016;11:20.
Lund H, Nielsen S, Grove A, et al. p57 in hydatidiform moles: evaluation of antibodies and expression in various cell types. Appl Immunohistochem Mol Morphol. 2020;28:694–701.
Zhang P, Zhu X, Yu X, et al. Abnormal processing of IL-1β in NLRP7-mutated monocytes in hydatidiform mole patients. Clin Exp Immunol. 2020;202:72–9.
Cozette C, Scheffler F, Lombart M, et al. Pregnancy after oocyte donation in a patient with NLRP7 gene mutations and recurrent molar hydatidiform pregnancies. J Assist Reprod Genet. 2020;37:2273–7.
Yu Y, Lu B, Lu W, et al. Whole-exome sequencing reveals genetic variants in ERC1 and KCNG4 associated with complete hydatidiform mole in Chinese Han women. Oncotarget. 2017;8:75264–71.
Reddy R, Nguyen NM, Sarrabay G, et al. The genomic architecture of NLRP7 is Alu rich and predisposes to disease-associated large deletions. Eur J Hum Genet. 2016;24:1445–52.
Kalogiannidis I, Kalinderi K, Kalinderis M, et al. Recurrent complete hydatidiform mole: where we are, is there a safe gestational horizon? Opinion and mini-review. J Assist Reprod Genet. 2018;35:967–73.
Nguyen NMP, Khawajkie Y, Mechtouf N, et al. The genetics of recurrent hydatidiform moles: new insights and lessons from a comprehensive analysis of 113 patients. Mod Pathol. 2018;31:1116–30.
Soper JT. Gestational trophoblastic disease: current evaluation and management. Obstet Gynecol. 2021;137:355–70.
Nguyen NMP, Ge ZJ, Reddy R, et al. Causative mutations and mechanism of androgenetic hydatidiform moles. Am J Hum Genet. 2018;103:740–51.
Mondal SK, Mandal S, Bhattacharya S, et al. Expression of p57 immunomarker in the classification and differential diagnosis of partial and complete hydatidiform moles. J Lab Physicians. 2019;11:270–4.
Banet N, DeScipio C, Murphy KM, et al. Characteristics of hydatidiform moles: analysis of a prospective series with p57 immunohistochemistry and molecular genotyping. Mod Pathol. 2014;27:238–54.
Oranratanaphan S, Khongthip Y, Areeruk W, et al. Determination of morphologic and immunohistochemical stain (p57 kip2) discrepancy of complete and partial hydatidiform mole by using microsatellite genotyping. Taiwan J Obstet Gynecol. 2020;59:570–4.
Creff J, Besson A. Functional versatility of the CDK inhibitor p57Kip2. Front Cell Dev Biol. 2020;8:584590.
White-Gilbertson S, Voelkel-Johnson C. Giants and monsters: unexpected characters in the story of cancer recurrence. Adv Cancer Res. 2020;148:201–32.
Stampone E, Bencivenga D, Barone C, et al. High dosage lithium treatment induces DNA damage and p57Kip2 decrease. Int J Mol Sci. 2020;21:1169.
Takahashi S, Okae H, Kobayashi N, et al. Loss of p57KIP2 expression confers resistance to contact inhibition in human androgenetic trophoblast stem cells. Proc Natl Acad Sci U S A. 2019;116:26606–13.
Eagles N, Sebire NJ, Short D, et al. Risk of recurrent molar pregnancies following complete and partial hydatidiform moles. Hum Reprod. 2015;30:2055–63.
Funding
This work was supported by grants from the Science Research Project of Anhui Provincial Health Commission (AHWJ2021b129) and the First Affiliated Hospital of Anhui University of Science and Technology Committee for Research and Conference Grant.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Fei Wang, Ping Zhou. (II) Administrative support: Jing Cheng. (III) Provision of study materials or patients: Fan Li. (IV) Collection and assembly of data: Ming-wei Li. (V) Data analysis and interpretation: Ming-wei Li and Fei Wang. (VI) Manuscript writing: Ming-wei Li and Fei Wang. (VII) Final approval of manuscript: all authors.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. The study was approved by the Local Ethical Committee ( no. ID RCB: 2020-Ethical review-14) before the first enrollment.
Consent to Participate
All patients gave their written consent to participate to this study.
Consent for Publication
All patients agreed for the publication of the data.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Li, Mw., Li, F., Cheng, J. et al. Recurrent Androgenetic Complete Hydatidiform Moles with p57KIP2-Positive in a Chinese Family. Reprod. Sci. 29, 1749–1755 (2022). https://doi.org/10.1007/s43032-021-00747-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00747-4