Abstract
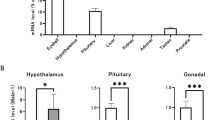
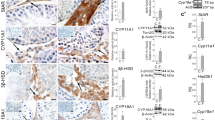
Testosterone, the male sex hormone, is necessary for the development and function of the male reproductive system. Biosynthesis of testosterone in mammals mainly occurs in testicular Leydig cells. Many proteins such as P450c17, 3β-HSD, and StAR are involved in testicular steroidogenesis. DAX1 is essential for sex development and interacts with nuclear receptors such as steroidogenic factor 1 to inhibit steroidogenesis. In this study, we investigated the role of DAX1 in testicular steroidogenesis in vivo by generating Leydig cell-specific DAX1-knockout mice. Radioimmunoassay revealed that the levels of testosterone and progesterone were higher in Leydig cell-specific DAX1-knockout testes than in the testes from wild-type mice during the first 3–4 weeks of aging. In addition, the expression levels of steroidogenic genes, such as StAR, P450c17, P450scc, and 3β-HSD, were considerably higher in the testes from DAX1-knockout mice. DAX1-deficient mouse testes seemed to attain early puberty with the acceleration of germ cell development. These data suggest that DAX1 regulates the expression of steroidogenic genes, and thereby controls and fine-tunes steroidogenesis during testis development.





Similar content being viewed by others
Availability of Data and Material
Not applicable
Code Availability
Prism software (Prism 5; GraphPad software, San Diego, CA, USA)
References
Franchimont P. Regulation of gonadal androgen secretion. Horm Res. 1983;18(1-3):7–17. https://doi.org/10.1159/000179774.
Stocco DM, Clark BJ. The role of the steroidogenic acute regulatory protein in steroidogenesis. Steroids. 1997;62(1):29–36.
Payne AH, Youngblood GL. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol Reprod. 1995;52(2):217–25. https://doi.org/10.1095/biolreprod52.2.217.
Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25(6):947–70. https://doi.org/10.1210/er.2003-0030.
Martin LJ, Tremblay JJ. Nuclear receptors in Leydig cell gene expression and function. Biol Reprod. 2010;83(1):3–14. https://doi.org/10.1095/biolreprod.110.083824.
Chanda D, Park J-H, Choi H-S. Molecular basis of endocrine regulation by orphan nuclear receptor small heterodimer partner. Endocr J. 2008;55(2):253–68. https://doi.org/10.1507/endocrj.K07E-103.
Iyer AK, McCabe ER. Molecular mechanisms of DAX1 action. Mol Genet Metab. 2004;83(1-2):60–73. https://doi.org/10.1016/j.ymgme.2004.07.018.
Ikeda Y, Takeda Y, Shikayama T, Mukai T, Hisano S, Morohashi KI. Comparative localization of Dax-1 and Ad4BP/SF-1 during development of the hypothalamic-pituitary-gonadal axis suggests their closely related and distinct functions. Dev Dyn. 2001;220(4):363–76. https://doi.org/10.1002/dvdy.1116.
Volle DH, Duggavathi R, Magnier BC, Houten SM, Cummins CL, Lobaccaro JM, et al. The small heterodimer partner is a gonadal gatekeeper of sexual maturation in male mice. Genes Dev. 2007;21(3):303–15. https://doi.org/10.1101/gad.409307.
Guo W, Burris TP, McCabe ER. Expression of DAX-1, the gene responsible for X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism, in the hypothalamic-pituitary-adrenal/gonadal axis. Biochem Mol Med. 1995;56(1):8–13. https://doi.org/10.1006/bmme.1995.1049.
Swain A, Zanaria E, Hacker A, Lovell-Badge R, Camerino G. Mouse Dax1 expression is consistent with a role in sex determination as well as in adrenal and hypothalamus function. Nat Genet. 1996;12(4):404–9. https://doi.org/10.1038/ng0496-404.
Ludbrook LM, Harley VR. Sex determination: a 'window' of DAX1 activity. Trends Endocrinol Metab. 2004;15(3):116–21. https://doi.org/10.1016/j.tem.2004.02.002.
Ikeda Y, Swain A, Weber TJ, Hentges KE, Zanaria E, Lalli E, et al. Steroidogenic factor 1 and Dax-1 colocalize in multiple cell lineages: potential links in endocrine development. Mol Endocrinol. 1996;10(10):1261–72. https://doi.org/10.1210/mend.10.10.9121493.
Babu PS, Bavers DL, Beuschlein F, Shah S, Jeffs B, Jameson JL, et al. Interaction between Dax-1 and steroidogenic factor-1 in vivo: increased adrenal responsiveness to ACTH in the absence of Dax-1. Endocrinology. 2002;143(2):665–73. https://doi.org/10.1210/endo.143.2.8658.
Hoyle C, Narvaez V, Alldus G, Lovell-Badge R, Swain A. Dax1 Expression is dependent on steroidogenic factor 1 in the developing gonad. Mol Endocrinol. 2002;16(4):747–56. https://doi.org/10.1210/mend.16.4.0802.
Lalli E, Sassone-Corsi P. DAX-1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Mol Endocrinol. 2003;17(8):1445–53. https://doi.org/10.1210/me.2003-0159.
Holter E, Kotaja N, Mäkela S, Strauss L, Kietz S, Jänne OA, et al. Inhibition of androgen receptor (AR) function by the reproductive orphan nuclear receptor DAX-1. Mol Endocrinol. 2002;16(3):515–28. https://doi.org/10.1210/mend.16.3.0804.
Agoulnik IU, Krause WC, Bingman WE 3rd, Rahman HT, Amrikachi M, Ayala GE, et al. Repressors of androgen and progesterone receptor action. J Biol Chem. 2003;278(33):31136–48. https://doi.org/10.1074/jbc.M305153200.
Lalli E, Ohe K, Hindelang C, Sassone-Corsi P. Orphan receptor DAX-1 is a shuttling RNA binding protein associated with polyribosomes via mRNA. Mol Cell Biol. 2000;20(13):4910–21. https://doi.org/10.1128/mcb.20.13.4910-4921.2000.
Zhang J, Liu G, Ruan Y, Wang J, Zhao K, Wan Y, et al. Dax1 and Nanog act in parallel to stabilize mouse embryonic stem cells and induced pluripotency. Nat Commun. 2014;5:5042. https://doi.org/10.1038/ncomms6042.
Lee SY, Park E, Kim SC, Ahn RS, Ko C, Lee K. ERalpha/E2 signaling suppresses the expression of steroidogenic enzyme genes via cross-talk with orphan nuclear receptor Nur77 in the testes. Mol Cell Endocrinol. 2012;362(1-2):91–103. https://doi.org/10.1016/j.mce.2012.05.015.
Suh JH, Gong EY, Hong CY, Park E, Ahn RS, Park KS, et al. Reduced testicular steroidogenesis in tumor necrosis factor-alpha knockout mice. J Steroid Biochem Mol Biol. 2008;112(1-3):117–21. https://doi.org/10.1016/j.jsbmb.2008.09.003.
Ahmed EA, de Rooij DG. Staging of mouse seminiferous tubule cross-sections. Methods Mol Biol. 2009;558:263–77. https://doi.org/10.1007/978-1-60761-103-5_16.
Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and Histopathological evaluation of the testis. 1st ed: Cache River Press; 1990.
Defalco T, Saraswathula A, Briot A, Iruela-Arispe ML, Capel B. Testosterone levels influence mouse fetal Leydig cell progenitors through notch signaling. Biol Reprod. 2013;88(4):91. https://doi.org/10.1095/biolreprod.112.106138.
Tremblay JJ. Molecular regulation of steroidogenesis in endocrine Leydig cells. Steroids. 2015;103:3–10. https://doi.org/10.1016/j.steroids.2015.08.001.
Muscatelli F, Strom TM, Walker AP, Zanaria E, Récan D, Meindl A, et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature. 1994;372(6507):672–6. https://doi.org/10.1038/372672a0.
Tabarin A. Congenital adrenal hypoplasia and DAX-1 gene mutations. Ann Endocrinol. 2001;62(2):202–6.
Bardoni B, Zanaria E, Guioli S, Floridia G, Worley KC, Tonini G, et al. A dosage sensitive locus at chromosome Xp21 is involved in male to female sex reversal. Nat Genet. 1994;7(4):497–501. https://doi.org/10.1038/ng0894-497.
Meeks JJ, Crawford SE, Russell TA, Morohashi K, Weiss J, Jameson JL. Dax1 regulates testis cord organization during gonadal differentiation. Development. 2003;130(5):1029–36. https://doi.org/10.1242/dev.00316.
Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL. Role of Ahch in gonadal development and gametogenesis. Nat Genet. 1998;20(4):353–7. https://doi.org/10.1038/3822.
Wang ZJ, Jeffs B, Ito M, Achermann JC, Yu RN, Hales DB, et al. Aromatase (Cyp19) expression is up-regulated by targeted disruption of Dax1. Proc Natl Acad Sci. 2001;98(14):7988–93. https://doi.org/10.1073/pnas.141543298.
Lalli E, Melner MH, Stocco DM, Sassone-Corsi P. DAX-1 blocks steroid production at multiple levels. Endocrinology. 1998;139(10):4237–43. https://doi.org/10.1210/endo.139.10.6217.
Zazopoulos E, Lalli E, Stocco DM, Sassone-Corsi P. DNA binding and transcriptional repression by DAX-1 blocks steroidogenesis. Nature. 1997;390(6657):311–5. https://doi.org/10.1038/36899.
Song KH, Park YY, Park KC, Hong CY, Park JH, Shong M, et al. The atypical orphan nuclear receptor DAX-1 interacts with orphan nuclear receptor Nur77 and represses its transactivation. Mol Endocrinol. 2004;18(8):1929–40. https://doi.org/10.1210/me.2004-0043.
McGee SR, Narayan P. Precocious puberty and Leydig cell hyperplasia in male mice with a gain of function mutation in the LH receptor gene. Endocrinology. 2013;154(10):3900–13. https://doi.org/10.1210/en.2012-2179.
Themmen AP. An update of the pathophysiology of human gonadotrophin subunit and receptor gene mutations and polymorphisms. Reproduction. 2005;130(3):263–74. https://doi.org/10.1530/rep.1.00663.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NRF-2017R1A2B4006166 and NRF-2020R1A2C1006705).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All the animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Chonnam National University (Permit Number: 2012-44).
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 14 kb).
Rights and permissions
About this article
Cite this article
Kumar, S., Kim, H.J., Lee, CH. et al. Leydig Cell–Specific DAX1-Deleted Mice Has Higher Testosterone Level in the Testis During Pubertal Development. Reprod. Sci. 29, 955–962 (2022). https://doi.org/10.1007/s43032-021-00554-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00554-x