Abstract
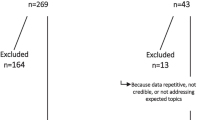
Conventional assisted reproductive technology (ART) cycles may delay cancer treatment and compromise survival, and also increase patients’ psychological burden as a result of delayed chemotherapy. The aim of this study was to compare the success rates of random start and conventional start GnRH antagonist protocols in terms of oocyte and embryo outputs in cancer patients. Data of 111 patients with a newly diagnosed cancer who underwent ART for fertility preservation at a university-based infertility clinic between January 2010 and September 2019 were reviewed. The study group underwent random start controlled ovarian hyperstimulation (RS-COH) and the control group underwent conventional start COH (CS-COH). The main outcome measures were the number of total oocytes, MII oocytes, and embryo yield. A total of 46 patients (41.5%) underwent RS-COH and 65 (58.5%) underwent CS-COH. Baseline characteristics were similar between the groups. The most common cancer type in both groups was breast cancer (60.9% vs. 52.3%, respectively). The median duration of stimulation was significantly longer in RS-COH than in CS-COH (12 vs. 10 days; P = 0.005). The median number of MII oocytes was significantly higher in RS-COH than in CS-COH (7 vs. 5 oocytes, respectively; P = 0.020). The MII/AFC ratio was significantly higher in the RS-COH group compared to the CS-COH group (74% and 57% respectively; p = 0.02). In the linear regression analyses, RS-COH protocol did not have a significant impact on MII/AFC (standardized ß coefficient − 0.514; P = 0.289 {adjusted R2 for the model = 0.779}), oocyte yield (standardized ß coefficient − 0.070; P = 0.829 {adjusted R2 for the model = 0.840}), and MII rate (standardized ß coefficient − 0.504; P = 0.596 {adjusted R2 for the model = 0.271}). In conclusion, RS-COH protocol is as effective as CS-COH protocols for fertility preservation in cancer patients.
Similar content being viewed by others
References
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–85.
Oktay K, Sonmezer M. Cryopreservation options for fertility preservation. UpToDate, last updated Jan 24, 2020. https://www.uptodate.com/contents/cryopreservation-options-for-fertility-preservation?search=Cryopreservation%20options%20for%20fertility%20preservation&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Accessed 02 Dec 2020.
Şükür YE, Özmen B, Sönmezer M. Cancer and ovarian tissue cryopreservation. Turk J Med Sci. 2010;40:159–68.
Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10:251–66.
Martinez F. Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Hum Reprod. 2017;32:1802–11.
Ethics Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org. Fertility preservation and reproduction in patients facing gonadotoxic therapies: an Ethics Committee opinion. Fertil Steril. 2018;110:380–6.
Anderson RA, Wallace WH. Fertility preservation in girls and young women. Clin Endocrinol. 2011;75:409–19.
von Wolff M, Montag M, Dittrich R, Denschlag D, Nawroth F, Lawrenz B. Fertility preservation in women--a practical guide to preservation techniques and therapeutic strategies in breast cancer, Hodgkin’s lymphoma and borderline ovarian tumours by the fertility preservation network FertiPROTEKT. Arch Gynecol Obstet. 2011;284:427–35.
Sönmezer M, Türkçüoğlu I, Coşkun U, Oktay K. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril. 2011;95:2125.e9–11.
Bedoschi G, Turan V, Emirdar V, Sonmezer M, Oktay KH. Comparison of random start controlled ovarian stimulation with standard start in letrozole gonadotropin cycles for fertility preservation in women with breast cancer. Fertil Steril. 2015;104:Suppl. e267.
Martínez F, Clua E, Devesa M, Rodríguez I, Arroyo G, González C, et al. Comparison of starting ovarian stimulation on day 2 versus day 15 of the menstrual cycle in the same oocyte donor and pregnancy rates among the corresponding recipients of vitrified oocytes. Fertil Steril. 2014;102:1307–11.
Seval MM, Özmen B, Atabekoğlu C, Şükür YE, Şimşir C, Kan Ö, et al. Dual trigger with gonadotropin-releasing hormone agonist and recombinant human chorionic gonadotropin improves in vitro fertilization outcome in gonadotropin-releasing hormone antagonist cycles. J Obstet Gynaecol Res. 2016;42:1146–51.
Ozkavukcu S, Celik-Ozenci C, Konuk E, Atabekoglu C. Live birth after Laser Assisted Viability Assessment (LAVA) to detect pentoxifylline resistant ejaculated immotile spermatozoa during ICSI in a couple with male Kartagener’s syndrome. Reprod Biol Endocrinol. 2018;16:10.
Alpha Scientists in Reproductive M, Embryology ESIGo. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83.
Gardner DK, Schoolcraft WB, Jansen R, Mortimer D. In vitro culture of human blastocysts, toward reproductive certainty: fertility and genetics beyond 1999. London: UK Parthenon Publishing; 1999. p. 378–88.
Danis RB, Pereira N, Elias RT. Random start ovarian stimulation for oocyte or embryo cryopreservation in women desiring fertility preservation prior to gonadotoxic cancer therapy. Curr Pharm Biotechnol. 2017;18:609–13.
de Mello Bianchi PH, Serafini P, Monteiro da Rocha A, Assad Hassun P, Alves da Motta EL, Sampaio Baruselli P, et al. Review: follicular waves in the human ovary: a new physiological paradigm for novel ovarian stimulation protocols. Reprod Sci. 2010;17:1067–76.
Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update. 2012;18:73–91.
Baerwald AR, Adams GP, Pierson RA. Characterization of ovarian follicular wave dynamics in women. Biol Reprod. 2003;69:1023–31.
Anderson RA, Kinniburgh D, Baird DT. Preliminary experience of the use of a gonadotrophin-releasing hormone antagonist in ovulation induction/in-vitro fertilization prior to cancer treatment. Hum Reprod. 1999;14:2665–8.
von Wolff M, Thaler CJ, Frambach T, Zeeb C, Lawrenz B, Popovici RM, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril. 2009;92:1360–5.
Bedoschi GM, de Albuquerque FO, Ferriani RA, Navarro PA. Ovarian stimulation during the luteal phase for fertility preservation of cancer patients: case reports and review of the literature. J Assist Reprod Genet. 2010;27:491–4.
Nayak SR, Wakim AN. Random-start gonadotropin-releasing hormone (GnRH) antagonist-treated cycles with GnRH agonist trigger for fertility preservation. Fertil Steril. 2011;96:e51–4.
Keskin U, Ercan CM, Yilmaz A, Babacan A, Korkmaz C, Duru NK, et al. Random-start controlled ovarian hyperstimulation with letrozole for fertility preservation in cancer patients: case series and review of literature. J Pak Med Assoc. 2014;64:830–2.
Farhi J, Orvieto R, Homburg R. Administration of clomiphene citrate in patients with polycystic ovary syndrome, without inducing withdrawal bleeding, achieves comparable treatment characteristics and outcome. Fertil Steril. 2010;93:2077–9.
Şükür YE, Özkavukçu S, İlhan FC, Şimşir C, Sönmezer M. Random start controlled ovarian hyperstimulation for fertility preservation during incidental pregnancy: a case report of blastocyst vitrification from in vitro matured oocytes. Gynecol Endocrinol. 2019;35:564–6.
Kuang Y, Hong Q, Chen Q, Lyu Q, Ai A, Fu Y, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril. 2014;101:105–11.
Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013;100:1673–80.
von Wolff M, Capp E, Jauckus J, Strowitzki T, Germeyer A, FertiPROTEKT study group. Timing of ovarian stimulation in patients prior to gonadotoxic therapy: an analysis of 684 stimulations. Eur J Obstet Gynecol Reprod Biol. 2016;199:146–9.
Nakasuji T, Kawai K, Ishikawa T, Teraoka K, Takeuchi S, Miyagawa T, et al. Random-start ovarian stimulation with aromatase inhibitor for fertility preservation in women with Japanese breast cancer. Reprod Med Biol. 2019;18:167–72.
Turan V, Bedoschi G, Moy F, Oktay K. Safety and feasibility of performing two consecutive ovarian stimulation cycles with the use of letrozole-gonadotropin protocol for fertility preservation in breast cancer patients. Fertil Steril. 2013;100:1681–5.e1.
Marklund A, Eloranta S, Wikander I, Kitlinski ML, Lood M, Nedstrand E, et al. Efficacy and safety of controlled ovarian stimulation using GnRH antagonist protocols for emergency fertility preservation in young women with breast cancer-a prospective nationwide Swedish multicenter study. Hum Reprod. 2020;35:929–38.
Alvarez RM, Ramanathan P. Fertility preservation in female oncology patients: the influence of the type of cancer on ovarian stimulation response. Hum Reprod. 2018;33:2051–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statements
Ethics committee approval was obtained from the Institutional Ethics Committee of Ankara University School of Medicine.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 20 kb).
Rights and permissions
About this article
Cite this article
İsrafilova, G., Şükür, Y.E., Özkavukcu, S. et al. Comparison of Oocyte and Embryo Quality Between Random Start and Controlled Ovarian Stimulation Cycles in Cancer Patients Undergoing Fertility Preservation. Reprod. Sci. 28, 2200–2207 (2021). https://doi.org/10.1007/s43032-020-00412-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00412-2