Abstract
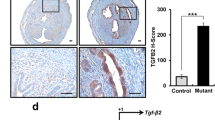
Adenomyosis is defined as the presence of endometrial glands and stroma in the myometrium. The mechanisms associated with the pathogenesis of adenomyosis remain unclear. Epithelial–mesenchymal transition (EMT) is characterized by losing cell polarity and cell–cell adhesion together with gaining migratory and invasive properties of stromal cells to become mesenchymal stem cells. Transforming growth factor-β1 (TGF-β1), an anti-inflammatory cytokine secreted by multiple cell types, plays a crucial role in embryogenesis and tissue homeostasis. The induction of EMT and ultimate fibrosis by TGF-β1 is suggested to play a critical role in the pathogenesis of adenomyosis. Thus, this study aims to demonstrate the occurrence of EMT in and the effects of anti-TGF-β1 on the pathogenesis of adenomyosis. ICR mice were fed with 1 μg/g body weight of tamoxifen (TAM) by in the first 4 postnatal days (PNDs). Subsequently, the right and left uterine horns were correspondingly injected with or without 10 μg of anti-TGF-β1 neutralizing antibody on PND42 followed by sacrifice on PND64. E-cadherin, vimentin, and α-smooth muscle actin (α-SMA) expression in the uteri was evaluated by qRT-PCR, Western blot, and immunohistochemistry. Clusters of endometrial glands and increased numbers of vimentin-positive stromal cells in the disrupted α-SMA-positive myometrium were observed in the uteri from TAM-treated mice. Numbers of stromal cells in the myometrium and the disrupted myometrial continuity were reduced by anti-TGF-β1. Moreover, uterine expression of E-cadherin and vimentin/α-SMA was increased and decreased by anti-TGF-β1 treatment, respectively. Anti-TGF-β1 successfully inhibits EMT and the development of adenomyosis in mouse uteri.






Similar content being viewed by others
References
Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):603–16. https://doi.org/10.1016/j.bpobgyn.2006.01.012.
Lazzeri L, Di Giovanni A, Exacoustos C, Tosti C, Pinzauti S, Malzoni M, et al. Preoperative and postoperative clinical and Transvaginal ultrasound findings of adenomyosis in patients with deep infiltrating endometriosis. Reprod Sci. 2014;21(8):1027–33. https://doi.org/10.1177/1933719114522520.
Li X, Liu X, Guo SW. Clinical profiles of 710 premenopausal women with adenomyosis who underwent hysterectomy. J Obstet Gynaecol Res. 2014;40(2):485–94. https://doi.org/10.1111/jog.12211.
Vannuccini S, Luisi S, Tosti C, Sorbi F, Petraglia F. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109(3):398–405. https://doi.org/10.1016/j.fertnstert.2018.01.013.
Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):511–21. https://doi.org/10.1016/j.bpobgyn.2006.01.016.
Parrott E, Butterworth M, Green A, White IN, Greaves P. Adenomyosis--a result of disordered stromal differentiation. Am J Pathol. 2001;159(2):623–30. https://doi.org/10.1016/S0002-9440(10)61733-6.
Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril. 2012;98(3):572–9. https://doi.org/10.1016/j.fertnstert.2012.06.044.
Leyendecker G, Bilgicyildirim A, Inacker M, Stalf T, Huppert P, Mall G, et al. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch Gynecol Obstet. 2015;291(4):917–32. https://doi.org/10.1007/s00404-014-3437-8.
Shaked S, Jaffa AJ, Grisaru D, Elad D. Uterine peristalsis-induced stresses within the uterine wall may sprout adenomyosis. Biomech Model Mechanobiol. 2015;14(3):437–44. https://doi.org/10.1007/s10237-014-0614-4.
Hufnagel D, Li F, Cosar E, Krikun G, Taylor HS. The role of stem cells in the etiology and pathophysiology of endometriosis. Semin Reprod Med. 2015;33(5):333–40. https://doi.org/10.1055/s-0035-1564609.
Moll R, Levy R, Czernobilsky B, Hohlweg-Majert P, Dallenbach-Hellweg G, Franke WW. Cytokeratins of normal epithelia and some neoplasms of the female genital tract. Lab Investig. 1983;49(5):599–610.
Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13(1):87–101. https://doi.org/10.1093/humupd/dml045.
Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22(2):137–63. https://doi.org/10.1093/humupd/dmv051.
Vannuccini S, Tosti C, Carmona F, Huang SJ, Chapron C, Guo SW, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod BioMed Online. 2017;35(5):592–601. https://doi.org/10.1016/j.rbmo.2017.06.016.
Carrarelli P, Yen CF, Arcuri F, Funghi L, Tosti C, Wang TH, et al. Myostatin, follistatin and activin type II receptors are highly expressed in adenomyosis. Fertil Steril. 2015;104(3):744–52 e1. https://doi.org/10.1016/j.fertnstert.2015.05.032.
Streuli I, Santulli P, Chouzenoux S, Chapron C, Batteux F. Activation of the MAPK/ERK cell-signaling pathway in uterine smooth muscle cells of women with adenomyosis. Reprod Sci. 2015;22(12):1549–60. https://doi.org/10.1177/1933719115589410.
Chen YJ, Li HY, Huang CH, Twu NF, Yen MS, Wang PH, et al. Oestrogen-induced epithelial-mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J Pathol. 2010;222(3):261–70. https://doi.org/10.1002/path.2761.
Oh SJ, Shin JH, Kim TH, Lee HS, Yoo JY, Ahn JY, et al. beta-Catenin activation contributes to the pathogenesis of adenomyosis through epithelial-mesenchymal transition. J Pathol. 2013;231(2):210–22. https://doi.org/10.1002/path.4224.
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73. https://doi.org/10.1038/nrc2620.
Zhang Q, Duan J, Liu X, Guo SW. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol Cell Endocrinol. 2016;428:1–16. https://doi.org/10.1016/j.mce.2016.03.015.
Shen M, Liu X, Zhang H, Guo SW. Transforming growth factor beta1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum Reprod. 2016;31(2):355–69. https://doi.org/10.1093/humrep/dev314.
Liu X, Shen M, Qi Q, Zhang H, Guo SW. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis. Hum Reprod. 2016;31(4):734–49. https://doi.org/10.1093/humrep/dew018.
Zhu B, Chen Y, Shen X, Liu X, Guo SW. Anti-platelet therapy holds promises in treating adenomyosis: experimental evidence. Reprod Biol Endocrinol. 2016;14(1):66. https://doi.org/10.1186/s12958-016-0198-1.
Zhang X, Zhang P, Shao M, Zang X, Zhang J, Mao F, et al. SALL4 activates TGF-beta/SMAD signaling pathway to induce EMT and promote gastric cancer metastasis. Cancer Manag Res. 2018;10:4459–70. https://doi.org/10.2147/CMAR.S177373.
Ling J, Cai Z, Jin W, Zhuang X, Kan L, Wang F, et al. Silencing of c-Ski augments TGF-b1-induced epithelial-mesenchymal transition in cardiomyocyte H9C2 cells. Cardiol J. 2019;26(1):66–76. https://doi.org/10.5603/CJ.a2018.0009.
Ungefroren H, Sebens S, Groth S, Gieseler F, Fandrich F. The Src family kinase inhibitors PP2 and PP1 block TGF-beta1-mediated cellular responses by direct and differential inhibition of type I and type II TGF-beta receptors. Curr Cancer Drug Targets. 2011;11(4):524–35.
Alipio ZA, Jones N, Liao W, Yang J, Kulkarni S, Sree Kumar K, et al. Epithelial to mesenchymal transition (EMT) induced by bleomycin or TFG(b1)/EGF in murine induced pluripotent stem cell-derived alveolar type II-like cells. Differentiation. 2011;82(2):89–98. https://doi.org/10.1016/j.diff.2011.05.001.
Pardali E, Sanchez-Duffhues G, Gomez-Puerto MC, Ten Dijke P. TGF-beta-induced endothelial-mesenchymal transition in fibrotic diseases. Int J Mol Sci. 2017;18(10). https://doi.org/10.3390/ijms18102157.
Johnson MC, Torres M, Alves A, Bacallao K, Fuentes A, Vega M, et al. Augmented cell survival in eutopic endometrium from women with endometriosis: expression of c-myc, TGF-beta1 and bax genes. Reprod Biol Endocrinol. 2005;3:45. https://doi.org/10.1186/1477-7827-3-45.
Young VJ, Ahmad SF, Duncan WC, Horne AW. The role of TGF-beta in the pathophysiology of peritoneal endometriosis. Hum Reprod Update. 2017;23(5):548–59. https://doi.org/10.1093/humupd/dmx016.
Inagaki N, Ung L, Otani T, Wilkinson D, Lopata A. Uterine cavity matrix metalloproteinases and cytokines in patients with leiomyoma, adenomyosis or endometrial polyp. Eur J Obstet Gynecol Reprod Biol. 2003;111(2):197–203.
Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164–85. https://doi.org/10.1016/j.jmig.2015.09.018.
Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, et al. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016;365(3):495–506. https://doi.org/10.1007/s00441-016-2464-0.
Dai C, Yang J, Liu Y. Transforming growth factor-beta1 potentiates renal tubular epithelial cell death by a mechanism independent of Smad signaling. J Biol Chem. 2003;278(14):12537–45. https://doi.org/10.1074/jbc.M300777200.
Dudas PL, Argentieri RL, Farrell FX. BMP-7 fails to attenuate TGF-beta1-induced epithelial-to-mesenchymal transition in human proximal tubule epithelial cells. Nephrol Dial Transplant. 2009;24(5):1406–16. https://doi.org/10.1093/ndt/gfn662.
Hu S, Yu W, Lv TJ, Chang CS, Li X, Jin J. Evidence of TGF-beta1 mediated epithelial-mesenchymal transition in immortalized benign prostatic hyperplasia cells. Mol Membr Biol. 2014;31(2–3):103–10. https://doi.org/10.3109/09687688.2014.894211.
Zhan M, Kanwar YS. Hierarchy of molecules in TGF-beta1 signaling relevant to myofibroblast activation and renal fibrosis. Am J Physiol Renal Physiol. 2014;307(4):F385–7. https://doi.org/10.1152/ajprenal.00338.2014.
Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med. 2015;21(9):998–1009. https://doi.org/10.1038/nm.3902.
Yanagita M. Inhibitors/antagonists of TGF-beta system in kidney fibrosis. Nephrol Dial Transplant. 2012;27(10):3686–91. https://doi.org/10.1093/ndt/gfs381.
Ji Y, Dou YN, Zhao QW, Zhang JZ, Yang Y, Wang T, et al. Paeoniflorin suppresses TGF-beta mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol Sin. 2016;37(6):794–804. https://doi.org/10.1038/aps.2016.36.
Feng H, Lu JJ, Wang Y, Pei L, Chen X. Osthole inhibited TGF beta-induced epithelial-mesenchymal transition (EMT) by suppressing NF-kappaB mediated Snail activation in lung cancer A549 cells. Cell Adhes Migr. 2017;11(5–6):464–75. https://doi.org/10.1080/19336918.2016.1259058.
McGaraughty S, Davis-Taber RA, Zhu CZ, Cole TB, Nikkel AL, Chhaya M, et al. Targeting anti-TGF-beta therapy to fibrotic kidneys with a dual specificity antibody approach. J Am Soc Nephrol. 2017;28(12):3616–26. https://doi.org/10.1681/ASN.2017010013.
Voelker J, Berg PH, Sheetz M, Duffin K, Shen T, Moser B, et al. Anti-TGF-beta1 antibody therapy in patients with diabetic nephropathy. J Am Soc Nephrol. 2017;28(3):953–62. https://doi.org/10.1681/ASN.2015111230.
Isaka Y. Targeting TGF-beta signaling in kidney fibrosis. Int J Mol Sci. 2018;19(9). https://doi.org/10.3390/ijms19092532.
Green AR, Styles JA, Parrott EL, Gray D, Edwards RE, Smith AG, et al. Neonatal tamoxifen treatment of mice leads to adenomyosis but not uterine cancer. Exp Toxicol Pathol. 2005;56(4–5):255–63. https://doi.org/10.1016/j.etp.2004.10.001.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. https://doi.org/10.1172/JCI39104.
Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyosis of the uterus--revisited. Am J Obstet Gynecol. 1972;112(5):583–93.
Skaland I, Janssen EA, Gudlaugsson E, Klos J, Kjellevold KH, Soiland H, et al. Phosphohistone H3 expression has much stronger prognostic value than classical prognosticators in invasive lymph node-negative breast cancer patients less than 55 years of age. Mod Pathol. 2007;20(12):1307–15. https://doi.org/10.1038/modpathol.3800972.
Kobayashi H, Kishi Y, Matsubara S. Mechanisms underlying adenomyosis-related Fibrogenesis. Gynecol Obstet Investig. 2019:1–12. https://doi.org/10.1159/000502822.
Akamatsu T, Arai Y, Kosugi I, Kawasaki H, Meguro S, Sakao M, et al. Direct isolation of myofibroblasts and fibroblasts from bleomycin-injured lungs reveals their functional similarities and differences. Fibrogenesis Tissue Repair. 2013;6(1):15. https://doi.org/10.1186/1755-1536-6-15.
Burns WC, Twigg SM, Forbes JM, Pete J, Tikellis C, Thallas-Bonke V, et al. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol. 2006;17(9):2484–94. https://doi.org/10.1681/ASN.2006050525.
Qi S, Zhao X, Li M, Zhang X, Lu Z, Yang C, et al. Aberrant expression of Notch1/numb/snail signaling, an epithelial mesenchymal transition related pathway, in adenomyosis. Reprod Biol Endocrinol. 2015;13:96. https://doi.org/10.1186/s12958-015-0084-2.
Jeong JH, Jang HJ, Kwak S, Sung GJ, Park SH, Song JH, et al. Novel TGF-beta1 inhibitor antagonizes TGF-beta1-induced epithelial-mesenchymal transition in human A549 lung cancer cells. J Cell Biochem. 2019;120(1):977–87. https://doi.org/10.1002/jcb.27460.
Nikitorowicz-Buniak J, Denton CP, Abraham D, Stratton R. Partially evoked epithelial-mesenchymal transition (EMT) is associated with increased TGFbeta signaling within lesional scleroderma skin. PLoS One. 2015;10(7):e0134092. https://doi.org/10.1371/journal.pone.0134092.
Guo S, Li Z, Yan L, Sun Y, Feng Y. GnRH agonist improves pregnancy outcome in mice with induced adenomyosis by restoring endometrial receptivity. Drug Des Devel Ther. 2018;12:1621–31. https://doi.org/10.2147/DDDT.S162541.
Lucarini L, Durante M, Lanzi C, Pini A, Boccalini G, Calosi L, et al. HYDAMTIQ, a selective PARP-1 inhibitor, improves bleomycin-induced lung fibrosis by dampening the TGF-beta/SMAD signalling pathway. J Cell Mol Med. 2017;21(2):324–35. https://doi.org/10.1111/jcmm.12967.
Feng T, Wei S, Wang Y, Fu X, Shi L, Qu L, et al. Rhein ameliorates adenomyosis by inhibiting NF-kappaB and beta-Catenin signaling pathway. Biomed Pharmacother. 2017;94:231–7. https://doi.org/10.1016/j.biopha.2017.07.089.
Chen Y, Zhu B, Zhang H, Liu X, Guo SW. Epigallocatechin-3-gallate reduces myometrial infiltration, uterine hyperactivity, and stress levels and alleviates generalized hyperalgesia in mice induced with adenomyosis. Reprod Sci. 2013;20(12):1478–91. https://doi.org/10.1177/1933719113488455.
Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015;125(7):2795–807. https://doi.org/10.1172/JCI77958.
Funding
This study was funded by E-Da hospital grants (EDAHP 106053 (NK), EDAHP107035 (NK), EDAHP108014 (NK), and EDAHP107015 (SJH).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kay, N., Huang, CY., Shiu, LY. et al. The Effects of Anti-TGF-β1 on Epithelial–Mesenchymal Transition in the Pathogenesis of Adenomyosis. Reprod. Sci. 27, 1698–1706 (2020). https://doi.org/10.1007/s43032-020-00139-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00139-0