Abstract
Mitochondrial activity is critical and correlates with embryo development. The identification of a novel human mitochondrial progesterone receptor (PR-M) that increases cellular respiration brings into question a role for progesterone in oocyte and preimplantation embryo development. Oocytes and embryos were generated from three Rhesus non-human primates (Macaca mulatta) undergoing in vitro fertilization. Immunohistochemical (IHC) staining for the progesterone receptor and mitochondria, RT-PCR with product sequencing for a mitochondrial progesterone receptor, and mitochondrial membrane determination with JC-1 staining were performed. IHC staining with selective antibodies to the progesterone receptor showed non-nuclear staining. Staining was absent in mouse control embryos. RT-PCR with product sequencing demonstrated PR-M transcript in Rhesus oocytes and embryos, which was absent in mouse embryos. Treatment of Rhesus oocytes and embryos with progesterone showed increased mitochondrial membrane potential, which was absent in mouse embryos. Our results support that progesterone increases mitochondrial membrane potential in oocytes and developing embryos. This is likely an in vivo mechanism to support preimplantation embryo development, and brings up the possibility of in vitro manipulation of culture media for optimization of growth.




Similar content being viewed by others
References
Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol. 2012;357:108–18.
Lydon J, DeMayo F, Funk C, Mani SK, Hughes AR, Montgomery CA Jr, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–78.
Shimada M, Yamashita Y, Ito J, Okazaki T, Kawahata K, Nishibori M. Expression of two progesterone receptor isoforms in cumulus cells and their roles during meiotic resumption of porcine oocytes. J Mol Endocrinol. 2004;33:209–25.
Peluso JJ, Romak J, Liu X. Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone’s antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations. Endocrinology. 2008;149:534–43.
Terzaghi L, Luciano AM, Dall’Acqua PC, Modina SC, Peluso JJ, Lodde V. PGRMC1 localization and putative function in the nucleolus of bovine granulosa cells and oocytes. Reproduction. 2018;155:273–82.
Saner K, Welter B, Zhang F, Hansen E, Dupont B, Wei Y, et al. Cloning and expression of a novel, truncated progesterone receptor. Mol Cell Endocrinol. 2003;200:155–63.
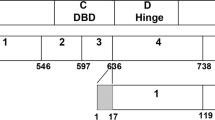
Dai Q, Shah AA, Garde RV, Yonish BA, Zhang L, Medvitz NA, et al. A truncated progesterone receptor (PR-M) localizes to the mitochondrion and controls cellular respiration. Mol Endocrinol. 2013;27:741–53.
Dai Q, Likes C, Luz A, et al. A mitochondrial progesterone receptor increase cardia beta-oxidation and remodeling. J Endocrine Soc. 2019;3(2):446–67.
Tantibhedhyangkul J, Hawkins KC, Dai Q, Mu K, Dunn CN, Miller SE, et al. Expression of a mitochondrial progesterone receptor in human spermatozoa correlates with a progestin-dependent increase in mitochondrial membrane potential. Andrology. 2014;2(6):875–83.
Feng Q, Crochet JR, Dai Q, Leppert PC, Price TM. Expression of a mitochondrial progesterone receptor (PR-M) in leiomyomata and association with increased mitochondrial membrane potential. J Clin Endocrinol Metab. 2014;99:E390–9.
Meyers S, Li M, Enders A, Overstreet J. Rhesus macaque blastocysts resulting from intracytoplasmic sperm injection of vacuum-dried spermatozoa. J Med Primatol. 2009;38:310–7.
Press M, Spaulding B, Groshen S, Kaminsky D, Hagerty M, Sherman L, et al. Comparison of different antibodies for detection of progesterone receptor in breast cancer. Steroids. 2002;67:799–813.
Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB. [29] Mitochondrial membrane potential monitored by JC-1 dye. In: GM Attardi (ed) Methods Enzymology, (vol: 260, pp. 406-17). Cambridge, Massachusetts, Academic Press.
Behera MA, Dai Q, Garde R, Saner C, Jungheim E, Price TM. Progesterone stimulates mitochondrial activity with subsequent inhibition of apoptosis in MCF-10A benign breast epithelial cells. Am J Physiol Endocrinol Metab. 2009;297:E1089–E96.
Petralia RS, Wang Y-X, Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol. 1994;349:85–110.
Dumollard R, Duchen M, Carroll J. The role of mitochondrial function in the oocyte and embryo. Curr Top Dev Biology, (pp. 21-49).Cambridge, Massachusetts, Academic Press.
Hou Q, Gorski J. Estrogen receptor and progesterone receptor genes are expressed differentially in mouse embryos during preimplantation development. Proc Natl Acad Sci U S A. 1993;90:9460–4.
Ying C, Yang Y-C, Hong W-F, Cheng W, Hsu W-L. Progesterone receptor gene expression in preimplantation pig embryos. Europ J Endocrinol. 2000;143:697–703.
Ahn K, Huh J-W, Park S-J, et al. Selection of internal reference genes for SYBR green qRT-PCR studies of rhesus monkey (Macaca mulatta) tissues. BMC Mol Biol. 2008;9:78.
Cossarizza A, Ceccarelli D, Masini A. Functional heterogeneity of an isolated mitochondrial population revealed by cytofluorometric analysis at the single organelle level. Exp Cell Res. 1996;222:84–94.
Mathur A, Hong Y, Kemp B, Barrientos A, Erusalimsky J. Evaluation of fluorescent dyes for the detection of mitochondrial membrane potential changes in cultured cardiomyocytes. Cardiovasc Res. 2000;46:126–38.
Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death. Eur J Biochem. 1999;264:687–701.
Duchen MR, Surin A, Jacobson J. Imaging mitochondrial function in intact cells. Methods Enzymol. 2003;361:353–89.
Zorov D, Juhaszova M, Sollott S. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–17.
Liu Y, Han M, Li X, Wang H, Ma M, Zhang S, et al. Age-related changes in the mitochondria of human mural granulosa cells. Hum Reprod. 2017;32:2465–73.
Reynier P, May-Panloup P, Chrétien MF, Morgan CJ, Jean M, Savagner F, et al. Mitochondrial DNA content affects the fertilizability of human oocytes. MHR: Basic Sci Reprod Med. 2001;7:425–9.
Duran HE, Simsek-Duran F, Oehninger SC, Jones HW, Castora FJ. The association of reproductive senescence with mitochondrial quantity, function, and DNA integrity in human oocytes at different stages of maturation. Fertil Steril. 2011;96:384–8.
Van Blerkom J, Davis P, Alexander S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: relationship to microtubular organization, ATP content and competence. Hum Reprod. 2000;15:2621–33.
Fragouli E, Spath K, Alfarawati S, et al. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015;11:e1005241.
Victor AR, Brake AJ, Tyndall JC, et al. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertil Steril. 2017;107:34–42.e3.
Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813.
Hardy K, Hooper M, Handyside A, Rutherford A, Winston R, Leese H. Non-invasive measurement of glucose and pyruvate uptake by individual human oocytes and preimplantation embryos. Hum Reprod. 1989;4:188–91.
Leese HJ, Baumann CG, Brison DR, McEvoy TG, Sturmey RG. Metabolism of the viable mammalian embryo: quietness revisited. MHR: Basic Sci Reprod Med. 2008;14:667–72.
Collado-Fernandez E, Picton H, Dumollard R. Metabolism throughout follicle and oocyte development in mammals. Int J Dev Biol. 2012;56:799–808.
Downs SM, Mosey JL, Klinger J. Fatty acid oxidation and meiotic resumption in mouse oocytes. Mol Reprod Dev. 2009;76:844–53.
Dunning K, Cashman K, Russell D, Thompson J, Norman R, Robker R. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod. 2010;83:909–18.
Matorras R, Ruiz JI, Mendoza R, Ruiz N, Sanjurjo P, Rodriguez-Escudero FJ. Fatty acid composition of fertilization-failed human oocytes. Hum Reprod. 1998;13:2227–30.
Leese H, Conaghan J, Martin K, Hardy K. Early human embryo metabolism. BioEssays. 1993;15:259–64.
Sturmey R, Leese H. Energy metabolism in pig oocytes and early embryos. Reprod. 2003;126:197–204.
McNatty KP, Smith DM, Makris A, Osathanondh R, Ryan KJ. The microenvironment of the human antral follicle: interrelationships among the steroid levels in antral fluid, the population of granulosa cells, and the status of the oocyte in vivo and in vitro *. J Clin Endocrinol Metab. 1979;49:851–60.
Maathuis J, Van Look P, Michie E. Changes in volume, total protein,and ovarian steroid concentrations of peritoneal fluid throughout the human menstrual cycle. J Endocrinol. 1978;76:123–33.
Acknowledgments
We thank Catherine VandeVoort, PhD, from the UC Davis California National Primate Research Center for providing cryopreserved Rhesus semen.
Funding
The Foundation of Assessment and Enhancement of Embryonic Competence, the Susan Fiery Hughes Memorial Research Foundation and the Charles B. Hammond, MD Research Fund
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, Q., Provost, M.P., Raburn, D.J. et al. Progesterone Increases Mitochondria Membrane Potential in Non-human Primate Oocytes and Embryos. Reprod. Sci. 27, 1206–1214 (2020). https://doi.org/10.1007/s43032-019-00132-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-019-00132-2