Abstract
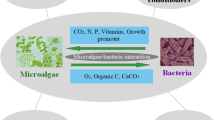
Products derived from microalgae have great potential in diverse field. As a part of the enhancing agriculture application, various forms of microalgae applications have been developed so far. They are known to influence soil properties. The various forms of application may enhance soil in more or less similar manner. They can help improve soil health, nitrogen, and phosphorus content, and even carbon sequestration. Thus, overall, it can enhance fertility of the soil.
Similar content being viewed by others
Introduction
Microalgae have captivated attention of many researchers in recent years due to their potential wide range of application (Spolaore et al. 2006). Some researcher mainly studies the production of biodiesel and other biofuels (bioethanol, biomethane, and biohydrogen) and generation of heat and electricity (Brennan and Owende 2010). While many studies the development of high value-added products from microalgae in areas such as nutrition and human health, aquaculture, cosmetics, and biofertilizers (Borowitzka. 2013). One of such implementations of Microalgae is in the field of agriculture considering the carbon and nitrogen fixing ability of some species.
Agricultural applications
Species like the Chlorophyte microalga has been proven to stabilize the soil and alters the hydrological properties (e.g., water retention) of crust covered soils in arid and semi-arid environments (Evans and Johansen 1999; Belnap and Lange 2003). Biological capture of carbon dioxide by using microalgae has shown promising, as microalgae fix CO2 during their growth (Wang et al. 2008; Douskova et al. 2009). Therefore, activities of microalgae improving soil functions and properties might be enhanced and exploited through applications of algal biomass as biofertilizers.
Apart from functions improving soil health, soil applications of microalgae may also serve for mitigation or sequestration of atmospheric carbon dioxide. Though autotrophic microorganisms are not generally thought to have a keyhole in CO2 fixation and sequestration in soils, global net carbon uptake of cryptogamic covers from the atmosphere amounts to ~3.9 Pg(Petagram) year−1, which is on a scale similar to the global annual carbon release due to biomass burning and fossil-fuel combustion, respectively (Elbert et al. 2012; Yuan et al. 2012).
Furthermore, microalgae are nutrient-rich, can store the inorganic nitrogen (N), Phosphorus (P) in excess within the cells in the form of protein and polyphosphate (Solovchenko et al. 2016) and thus own potential to transformed from biowaste to biofertilizer (Ray et al. 2013; Mukherjee et al. 2015; Santos and Pires, 2018.). Chlorella, a green microalga, contains significant quantities of N and P (up to 7–12% and 1–3% of their cell dry weight, respectively) (Powell et al. 2009; Cabanelas et al. 2013; Zhu et al. 2015). Hence, microalgae have the capacity to be used as cost effective, environmentally friendly, and sustainable alternative biofertilizer to traditional chemical fertilizer as they not only increase agricultural production but also minimizes the negative environmental impact on land use (Kawalekar 2013; Sigurnjak et al. 2017). Biofertilizer prevents loss of nutrients and can supply almost all the nutrients required for plant growth (Kokare et al. 2015) in the absences of chemical fertilizers, the crop also depends entirely on the mineralization of organically bound nutrients within the biofertilizers.
However, direct application of microalgae did not deliver significant difference on the growth of wheat (Schreiber et al. 2018) or rice (Ray et al. 2013; Mukherjee et al. 2015) because the dominant forms of the stored N and P in microalgae are proteins and polyphosphates that are difficult to decompose in soil and unable to be directly utilized by plants. So, several utility forms have been employed.
Microalgae forms for soil application
One such form is the hydrothermal carbonization (HTC), which can transform the microalgae biomass into hydrochars. This is recommended as it has been demonstrated to transform most polyphosphates and proteins from ligno-cellulosic feedstock (Funke et al. 2013; Kruse et al. 2016) and biosolids (Huang and Tang 2015; Huang et al. 2017;** Yu et al. 2019) into orthophosphate and ammonium or nitrate. HTC is cost-effective to avoid dehydrating the microalgae collected from the wastewater. Generally, biochar application can reduce soil pH and increase porosity, aeration, and redox potential, thus reducing NH3. Citrate acid was added to increase the hydrochar yield (Heilmann et al. 2010) reduce hydrochar pH and promote the degradation of proteins and polyphosphates by acidic hydrolysis (Huang et al. 2017).
Another form of application is algal biofertilizer. Biofertilizers have emerged as best alternative to synthetic fertilizers. A biofertilizer comprises living microorganisms, which on application colonizes the rhizosphere or the interior of the plant, plant let or seed surfaces or soil, thus promoting growth by accelerating the availability of primary nutrients to the host plant. Biofertilizers comprise of microorganisms, including bacteria, fungi, cyanobacteria, and algae as well as their metabolites that can enhance soil, crop growth, and yield. Biofertilizers include symbiotic nitrogen fixers like Rhizobium spp. associated with leguminous crops, non-symbiotic free-living nitrogen fixers like Azotobacter, which can be used for crops like maize, wheat, cotton, mustard, potato, and other vegetable crops, while Azospirillium is mainly used for sorghum, millets, maize, sugarcane, and wheat. Algal biofertilizers like the cyanobacteria such as Nostoc sp., Anabaena sp., Tolypothrix sp., Aulosira sp. etc., have the potential to fix atmospheric nitrogen and are used in paddy fields. Some other types include mycorrhizae, organic fertilizers, and phosphate-solubilizing bacteria. Pantoea agglomerans, one of the phosphate-solubilizing bacteria such as strain P5, and Pseudomonas putida strain P13 can solubilize the insoluble phosphate from organic and inorganic sources. The Azolla-Anabaena and Rhizobium form the most important group of biofertilizers. Biofertilizers have numerous benefits to soil quality and crop yield as they enhance nutrient transfer, increase population of beneficial microorganisms, stabilize soil aggregates, and decrease reliance on fossil fuels. Benefits of different microalgal-biofertilizers are listed in Table 1.
A new emerging application is the harvest and utilization of algal biomass produced in waste treatment since microalgae may produce bioactive substances such as phytohormones or accumulate elements of interest (Mallick 2002; Stirk and Van Staden 2010). Aerobic soil applications with living algae present an entirely different scenario: microalgae are specifically adapted to aquatic environments, requiring continuous hydration for maintenance of cellular structure, nutrient acquisition, and gas diffusion. As such, in soil environments, the proliferation of microalgae is strongly conditional by humidity, and the temporal desiccation will occur in arid and semi-arid environments where the benefits of soil organic matter may be greatest. Despite these obvious constraints, microalgae, alone or as a component of biological soil crusts (BSC) play roles in soil nutrient cycling and water fluxes (Maestre et al. 2011).
The de-oiled microalgal biomass waste (DOMBW), which contains a high amount of nitrogen, phosphorus, potassium, and other nutrients, can serve as a biofertilizer (Dineshkumar et al. 2018). Microalgae-based biofertilizer has the capability to decrease nutrient losses through a consistent release of nutrient, which can suitably fulfil the nutrient requirements of the crops (Renuka et al. 2018). Besides macronutrients, microalgae also contain trace elements and natural phytohormones, which are essential for proper growth and development of plants (Dineshkumar et al. 2018). Using N-rich biomass of microalgae as biofertilizer also have additional benefits such as carbon sequestration, improved soil health, soil water retention, stability of soil aggregates, and prevention of nutrient losses (Sole-Bundo et al. 2017) therefore, DOMBW is also a promising source of biofertilizer.
Implemented in photobioreactors, microalgae can metabolize and accumulate residual nutrients from agro-industrial waste (Morales-Amaral et al. 2015). They have potential to increase soil organic matter content and fix additional atmospheric C into Croplands need to be tested. Soil organic matter can correlate with P adsorption both positively mainly due to the anionic character of organic matter, and negatively, blocking P adsorption sites in soil (Novais et al. 2007). Therefore, it should be stated that each material, whether animal waste, biochar, or any biomass rich in organic matter may, by virtue of its constitution behave differently when added to the fertilizer mass. In this context, the possibility of microbial biomass grown in wastewaters may also be added to phosphate fertilizers to increase the adsorption efficiency of P by plants. There are few published works referring to these theme (Castro et al. 2017; Marks et al. 2017) and survey that evaluate the environmental impact of the use of this material as a source of nutrient are still incipient.
Different processes of preparation
-
1.
Microalgae Biofertilizer (Castro et al. 2020):
To be used as biofertilizer, they need to undergo two production steps, viz. cultivation, and processing. They are explained as below:
Algae Biomass cultivation:
-
(a)
Multiplication
A High-Rate Algal Pond (HRAP) (area = 3.30 m2 & volume=1 m3) was operated in batch mode (14 days of operation) to produce MB. The HRAP had a six-blade stainless steel paddlewheel, powered by a 1 HP electric motor responsible for operating 12 ponds. During the operation, the CO2 supplementation was controlled from the pH variation in the units. In addition, it had a CO2 injection system, in which a gas cylinder injection system, in which a gas cylinder containing 99% CO2 and a pump were used to recirculate the effluent in the carbonation column.
-
(b)
Coagulation
After the production phase, the biomass was treated with 505 mV s−1(millivolt per second) sodium hydroxide (NaOH), promoting a pH increase up to 12. And then for coagulation, a suitable hydraulic gradient was generating by moving a paddlewheel for nearly 2 h.
-
(c)
Collection
Biomass was collected after resting the effluent inside the HRAP for 24h. The main characteristics of the biomass at the end of the batch were as follows: volatile suspended soils = 571.81 mg L−1(milligram per litre); total phosphorus = 7.40 mg L−1 and total nitrogen = 68.40 mg L−1.
-
(d)
Drying
The biomass was dried in a forced circulation greenhouse (3 kwh) for 2 days. The greenhouse has drying capacity up to 400 kg of biomass at a time.
-
(a)
Biofertilizer processing
The biofertilizer was produced by granulation process, with the addition of 12% dry MB (Microalgae Biomass) into triple superphosphate (TSP), Ca(H2PO4)2.H2O. This proportion was chosen, after previous experiment conducted by Castro et al. 2020. Authors tested several other addition proportions and 12% of MB corresponds to the value that presented higher P content in the millet plant shoot (Pennisetum glaucum L.) TSP + 12% MB showed no difference in P diffusion in the soil, while increase in proportion above 30% MB clearly impaired P diffusion.
In the processing, first the inoculum was maintained in 2L borosilicate bioreactors using sterilized medium, 3N-BBMV (Bold’s Basal medium with vitamins and triple nitrate).
Operational conditions were as follows: constant aeration using 2.5% CO2 at 0.2 Vvm (Volume of gas per volume of culture per minute), a photoperiod of 14:10 light: dark cycle, 150 µmol m−2 s−1 of luminance and temperature of 25 ± 1 °C.
The microalgal cells were then collected from a cultivation broth using centrifugation of 8000g for 5 min at 4 °C, followed by washing using distilled water and finally freeze-dried.
Hydrothermal carbonization (HTC) was conducted in a high-pressure (approximate 8 MPa, autogenerated during HTC) hydrothermal reactor. The process begins by loading the microalgae into the reactor. The reactor was sealed and heated at 260 °C for 1 h and then allowed to naturally cool down to room temperature overnight. The solid hydrochars produced by HTC were collected by centrifugation and dried at 70 °C until no further weight loss. Two hydrochars that had been produced by using different reaction media viz. CVHW, Chlorella vulgaris-derived hydrochars with water (employing deionized water) and CVHCA Chlorella vulgaris-derived hydrochars with citric acid (employing 1 wt% citric acid). Citric acid was added to increase the hydrochar yield (Heilmann et al. 2010), reduce hydrochar pH and promote the degradation of proteins and polyphosphates by acidic hydrolysis (Huang et al. 2017).
The microalga was cultivated in an open raceway pond with a working volume of 60 L for 7 days in batch mode, where the solar intensities varied between 300 lx to 48000 lx. The temperature ranged from 27 °C to 33 °C and the average relative humidity varied from 55% to 92%. The culture was grown using domestic wastewater as the growth medium and supplemented with coal-fired flue gas (2.5% CO2) as the carbon source. The different nutrient components present in the wastewater were as follows: ammonium (NH4+ –N) 38.6 mg L−1 Nitrate (NO3−–N) 17.1 mg L−1, Phosphate (PO4−3–P) 9.24 mg L−1 and chemical oxygen demand (COD) 142.2 mg L−1The flue gas comprised of CO2 12% (v/v), carbon monoxides (CO) 0.55% (v/v) sulfur dioxides (SO2) 0.3% (v/v) and nitrogen oxides (NOx) 61 ppm.
After cultivation, the culture was concentrated via flocculation using chitosan as flocculant. The upper medium layer was decanted, and the lower concentrated biomass phase was dried under direct sunlight. The organic cationic flocculant, chitosan was used to coagulate negatively charged microalgae cell to avoid high energy centrifugation method. The dried algal biomass is then used for the extraction of oil. the residual de-oiled biomass was dried at 70 °C for 48 h to remove the solvent, and it was stored at −80 °C until later use. For use as a fertilizer, the DOMBW was mixed into soil and used for the cultivation.
-
(4)
Microalgae slurry (Marks et al. 2017):
Live algae collected or produced by earlier mentioned biomass production technique are used as a liquid slurry. No further processing is to be done. Those liquid slurry are used directly in field.
Influences of microalgae on soil properties
Even though the form of microalgae applied differs, their fate in soil is identical, i.e., their effect on the soil may be almost similar with negligible differences. So, their effect on soil has been clubbed together and discussed under the following subheadings.
Soil chemical properties
Soil pH is also known to be affected by algal application. Saha and Mandal (1979) reported an initial increase in soil pH, whereas contradictory to it Subhashini and Kaushik (1981) reported a significant reduction not only in pH but also in hydraulic conductivity, electrical conductivity (EC), and soil aggregation. cyanobacteria are also known for their ability to release trace elements from insoluble materials. Fe, Mn, and Zn are known to be influenced in rice fields by cyanobacterial growth (Das et al. 1991). Lange (1976) reported chelation of Fe, Cu, Mo, Zn, Co, and Mn through gelatinous sheath of many cyanobacterial species. This sheath is also known to reduce particle erosion and may adsorb charged nutrient cations (Whitton 2000). In summary, algal application influence soil properties through soil particle aggregation, phosphate and trace element release from insoluble minerals, and N storage and its slow release. The chlorella algae grown in an inert substrate can fix 0.5 mg of CO2–C over the test period. Chlorella has greater effect of C fixation than the native algae.
Chlorella microalgae applied as powder or as hydrochar employing water or citrate solution, significantly improved the soil NH4+ concentration also improving NO3–N concentration by 46.5%. Application of Chlorella in different forms affect the pH of soil, being consistent with pH of the material.
Nayak et al. (2016) reported a significantly lower pH value of the soil with supplementation of chemical fertilizer in comparison with those treated with de-oiled microalgae biomass waste (DOMBW). The increased value can be due to the release of NH4+–N form protein degradation of biomass. It was also found that supplementation of DOMBW results in significantly higher EC value of the soil in comparison to the application of other organic fertilizer at both tillering and harvesting stage of growth. High EC values shows continues availability of soluble nutrients in the form of both cations and anions to support healthy growth and development of plants especially rice (Eigenberg et al. 2002; Meng et al. 2018).
The nutrient availability was found to be optimum for proper growth of rice when soil was supplemented with algal-based fertilizer in comparison to chemical fertilizer or vermicompost supply. The likely explanation is that the DOMBW requires time to be decomposed into usable nutrients, which means that the nutrients are released steadily throughout the crop cultivation (Castro et al. 2017). A similar observation was also observed by Renuka et al. (2016), where available N, P, and K was found to be increased when microalgae was supplied.
Effect on soil physical properties
Effect of surface growth of inoculated cyanobacteria on subsurface properties of a brown earth, silt loam soil was studied by Rao and Burns (1991). Significant increase in soil polysaccharides, dehydrogenase, urease, and phosphatase activities was recorded. Improvement in soil aggregation was also seen; stable soil aggregates are essential to soil fertility. Studies of Burns and Davics (1986) suggested soil polysaccharides as major component responsible for soil stabilization. However, these effects were confined to surface layer of 0–0.7 cm depth. The results of Roychoudhury et al. (1979) also demonstrated improvement in soil aggregation.
Inoculations with cyanobacteria provide a better water holding capacity in the soil. These were supported by several other studies (Singh 2008; Bailey et al. 1973; de Winder et al. 1989). The improvement in soil aggregation was due to Algal proteoglycans which possess adhesive properties, and easily fasten cells to solid surfaces (Flaibani et al. 1989). Soil aggregation and arrangement of the soil aggregates are important as it directly affects temperature, aeration, and infiltration rates of the soil, which ultimately improves the physical environment of the crop (Falchini et al. 1996). Furthermore, besides soil aggregation, soil porosity is also enhanced by inoculating Nostoc strains on clay soils (Falchini et al. 1996). There are reports suggesting solubilization of insoluble forms of inorganic phosphate by cyanobacterial inoculation (Singh 2008; Kleiner and Harper 1977). It was further evidenced by studies of Bose et al. (1971), Cameron and Julian (1988), and Roychoudhury and Kaushik (1989), which advocated cyanobacterial phosphorous solubilizing activity on hydroxyapatite, tricalcium phosphate, and Mussorie rock phosphate. Apart from phosphorous, there are several evidences that witnessed an increase in N content and organic matter of soils inoculated with cyanobacteria (Singh and Singh 1989; Vaishampayan et al. 2001; Venkataraman 1993). Castro et al. (2020) studied that use of microalgae as biofertilizer along with chemical fertilizer is an efficient and viable option for improving and restoring soil fertility along with superior crop productivity.
Effect on soil biological properties
Although studies have been undergoing on the effect of algal biofertilizer on soil microflora, limited details are known about the associative changes in soil microbial community following inoculation with cyanobacteria or other algae. The application of a photosynthetic algal suspension increased eukaryotic and prokaryotic biomass and the activities of heterotrophic microorganisms in the soil. Rao and Burns (1991) reported an eightfold increase in bacterial members in the cyanobacteria inoculated columns, whereas increase in fungal population was not significant. Ibrahim et al. (1971) reported an increase in total microbial community in a pot experiment specifically nitrifiers (genera of Azotobacter and Clostridium) after inoculation of Tolypothrix tenuis. Acea et al. (2001) reported greater than four logarithmic unit increases in heterotrophic bacteria, actinomycetes, algal, and fungal propagules and three logarithmic unit increases in fungal mycelia after inoculating burnt soils with cyanobacteria. Similarly, Rogers and Burns (1994) reported a significant difference in the heterotrophic microbial population after inoculation of soil with Nostoc muscorum. These results suggest additional carbon and energy source due to cyanobacterial polysaccharides as one of the reasons behind increase in heterotrophic microbial populations. Increment in total nitrogen content of inoculated soil also stimulates indigenous soil microorganisms. Reports of Chu et al. (2020a, b) shows the application of Chlorella vulgaris powder and hydrochars had marked impacts on the activities of soil microorganisms that are responsible for nitrification and denitrification. Nutrient status of soil specifically nitrogen and phosphorous determines the mineralization of available carbon and thus affects the microbial community (Anderson and Gray, 1991).
Conclusions
Thus, from the above acknowledge information, we know that microalgae have high capability of improving the soil properties. Even though some findings are still uncertain like how much impact it can have on other microbial population under long-term application or how efficient it can be as compared with the inorganic sources. It is evident that the effect on soil properties has been successful so far though the results are based on short-term research. Since major farming population has already witness the immense impact of excessive use of chemicals on soil health leading to soil degradation, an organic supplement is needed to reduce its use. Addition of microalgae can be one such useful method to minimize the negative effects of excessive chemical use. Research on the application of microalgae in agriculture is still in its early stages and has yet to be tested on a large scale.
References
Acea MJ, Diz N, Prieto-Fernández A (2001) Microbial populations in heated soils inoculated with cyanobacteria. Biol Fert Soils 33:118–125
Anderson TH, Gray TRG (1991) The influence of soil organic carbon on microbial growth and survival. In: Wilson WS (ed) Advances in Soil Organic Matter Research: The Impact on Agriculture and the Environment. Royal Society of Chemistry, Cambridge, pp 253–266
Bailey DA, Mazurak AP, Rosowski JR (1973) Aggregation of soil particles by algae. J Phycol 9:99–101
Belnap J, Lange O (2003) Structure and functioning of biological soil crusts: synthesis. In: Belnap J, Lange O (eds) Biological Soil Crusts: Structure, Function, and Management. Springer-Verlag, Berlin, pp 471–479
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756. https://doi.org/10.1007/s10811-013-9983-9.
Bose P, Nagpal US, Venkataraman GS, Goyal SK (1971) Solubilization of tricalcium phosphate by blue-green algae. Curr Sci 7:165–166
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577. https://doi.org/10.1016/j.rser.2009.10.009
Burns RG, Davics JA (1986) The microbiology of soil structure. Biol Agric Hortic 3:95–113
Cabanelas ITD, Ruiz J, Arbib Z, Chinalia FA, Garrido-Pirez C, Rogalla F, Nascimento IA, Perales JA (2013) Comparing the use of different domestic waste-waters for coupling microalgal production and nutrient removal. Bioresour Technol 131:429–436. https://doi.org/10.1016/biotech.2012.12.152
Cameron HJ, Julian GR (1988) Utilization of hydroxyapatite by cyanobacteria as their sole source of phosphate and calcium. Plant Soil 109:123–124
Castro JS, Calijuri ML, Assemary PP, Cecon PR, Assis IR, Ribeiro (2017) Microalgae biofilm in soil:greenhouse gas emissions, ammonia volatilization and plant growth. Sci Total Environ 574:1640–1648
Castro JS, Calijuri ML, Ferreira J, Paula PA, Vinícius JR (2020) Microalgae based biofertilizer: A life cycle approach. Sci Total Environ 724. https://doi.org/10.1016/j.scitotenv.2020.138138
Chatterjee A, Singh S, Agrawal C, Yadav S, Rai R, Rai LC (2017) Role of Algae as a biofertilizer. Algal Green Chem, pp 189–197. https://doi.org/10.1016/B978-0-444-63784-0.00010-2
Chu Q, Xue L, Cheng Y, Liu Y, Feng Y, Yu S, Meng L, Pan G, Hou P, Duan J, Yang L (2020) Microalgae-derived hydrochar application on rice paddy soil. Higher rice yield but increased gaseous nitrogen loss. Sci Tot Environ. https://doi.org/10.1016/j.scitotenv.2020.137127
Qingnan Chu, Lihong Xue, Yueqin Cheng, Yang Liu, Yanfang Feng, Shan Yu, Lin Meng , Gang Pan, Pengfu Hou, Jingjing Duan, Linzhang Yang (2020. Microalgae-derived hydrochar application on rice paddy soil: Higher rice yield but increased gaseous nitrogen loss. Sci Total Environ 717:137127.
Das SC, Mandal M, Mandal LN (1991) Effect of growth and subsequent decomposition of blue-green algae on the transformation of iron and manganese in submerged soils. Plant Soil 138:75–84
De PK (1939) The role of blue-green algae in nitrogen fixation in rice fields. Proc R Soc B 127:121–213
de Winder B, Pluis J, de Reus LR (1989) Morphological characterization of a cyanobacterial, algal dune crust in the coastal dunes of The Netherlands. In: Cohen Y, Rosenberg E (eds) Microbial Mats. Physiological Ecology of Benthic Microbial Communities, American Society for Microbiology, Washington, DC, pp 77–83
Dineshkumar R, Kumaravel R, Gopalsamy J, Sikder MNA, Sampathkumar P (2018) Microalgae as bio-fertilizers for rice growth and seed yield productivity. Waste Biomass Volariz 9:793–800
Douskova I, Doucha J, Livansky K, Machat J, Novak P, Umysova D, Zachleder V, Vitova M (2009) Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl Microbiol Biotechnol 82:179–185. https://doi.org/10.1007/s00253-008-1811-9
Eigenberg RA, Doran JW, Nienaber JA, Ferguson RB, Woodbury BL (2002) Electrical conductivity monitoring of soil condition and available N with animal manure and a cover crop. Agric Ecosyst Environ 88:183–193
Elbert W, Weber B, Burrows S, Steinkamp J, Büdel B, Andreae MO, Pöschl U (2012) Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat Geosci 5:459–462
Evans RD, Johansen JR (1999) Microbiotic crusts and ecosystem processes. Crit Rev Plant Sci 18:183–225
Falchini L, Sparvoli E, Tomaselli L (1996) Effect of Nostoc (cyanobacteria) inoculation on the structure and stability of clay soils. Biol Fert Soils 23:246–252
Flaibani A, Olsen Y, Painter TJ (1989) Polysaccharides in desert reclamation: composition of exocellular proteoglycan complexes produced by filamentous blue-green and unicellular green edaphic algae. Carbohydr Res 190:235–248
Funke A, Mumme J, Koon M, Diakte M (2013) Cascaded production of biogas and hydrochar from wheat straw: energetic potential and recovery of carbon and plant nutrients. Biomass Bioenergy. https://doi.org/10.1016/j.biombioe.2013.08.18
Heilmann SM, Davis HT, Jader LR, Lefebere PA, Sodowsky MJ, Schendel FJ, Von Keitz MG, Valentas KJ (2010) Hydrothermal carbonization of microalgae. Biomass Bioenergy 34:875–882. https://doi.org/10.1016/j.biombioe.2010.01.032
Huang R, Tang Y (2015) Speciation dynamics of phosphorus during (hydro) thermal treatments of sewage sludge. Environ Sci Technol 48:14466–14474. https://doi.org/10.1021/acs.est.5b04140
Huang R, Fang C, Lu X, Jiang R, Tang Y (2017) Transformation of phosphorus during (hydro) thermal treatments of solid biowastes: reaction mechanisms and implications for P reclamation and recycling environment. Sci Technol. https://doi.org/10.1021/acs.est.7b02011
Ibrahim AN, Kamel M, El-sherbeny M (1971) Effect of inoculation of alga Tolypothrix tenuis on the yield of rice and soil nitrogen balance. Agrokem Talajtan 20:389–400
Kawalekar SJ (2013) Role of biofertilizers and biopesticides for sustainable agriculture. J Biol Innov 2:73–78
Kleiner KT, Harper KT (1977) Soil properties in relation to cryptogamic ground cover in Canyonlands National Park. J Range Manage 30:202–205
Kokare VG, Kasture MC, Palsande VN, Mhalshi RM (2015) Effect of different fertilizer briquettes and organic manures on yield, nutrients uptake and chemical properties of soil in chilli (Capsicum annum) in lateritic soils of Konkan. Int J Agri Sci Res 5:13–18
Kruse A, Koch F, Stelzl K, Wüst D, Zeller M (2016) Fate of nitrogen during hydrothermal carbonization. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.6b01312
Lange W (1976) Speculations on a possible essential function of the gelatinous sheath of blue-green algae. Can J Microbiol 22:1181–1185
Maestre FT, Boroker MA, Cantòn Y, Castillo-Monroy AP, Cortina J, Escolar C, Escudero A, Lẚzaro R, Martinez I (2011) Ecology and functional roles of biological soil crusts in semi-arid ecosystems of Spain. J Arid Environ 75:1282–1291
Mallick N (2002) Biotechnological potential of immobilized algae for wastewater N. P and metal removal: a Review. Biometals 15:377–390
Marks EAN, Miňoń J, Pascual A, Montero O, Navas LM, Rad C (2017) Application of a microalgal slurry to soil stimulates hetetrophic activity and promotes bacterial growth. Sci Total Environ 605–606:610–617
Meng J, Tao M, Wang L, Liu X, Xu J (2018) Changes in heavy metal bioavailability and speciation from a Pb-Zn mining soil amended with biochars from co-pyrolysis of rice straw and swine manure. Sci Total Environ 633:300–307
Moore AW (1969) Azolla: biology and agronomic significance. Bot Rev 35:17–34
Morales-Amaral M, Gòmez- Serrano C, Fernandez-Sevilla JM, Molina-Grima E (2015) Outdoor production of Scenedesmus sp. in thin-layer and raceway reactors using centrate from anaerobic digestion digestion as the sole nutrient source. Algal Res 12:99–108
Mukherjee C, Chowdhury K, Ray K (2015) Phosphorus recycling from an unexplored source by polyphosphate accumulating micro algae and cyanobacteria-a step to phosphorus security in agriculture. Front Microbial 6:1–7. https://doi.org/10.3389/fmicb.2015.01421
Nayak M, Karemore A, Sen R (2016) Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis foe biodiesel application. Algal Res 16:216–223
Nayak M, Dillip KS, Ramkrishna S (2019a) Strategic valorization of de-oiled microalgal biomass waste as biofertilizer for sustainable and improved agriculture of rice (Oryza sativa L.) crop. Sci Total Environ 682:475–484
Nayak M, Rashid N, Suh WL, Lee B, Chang YK (2019b) Performance evaluation of different cationic flocculants through pH modulation for efficient harvesting of Chlorella sp. HSZ and their impact on water reusability. Renew Energy 136:819–827
Novais RF, Smyth TJ, Nunes FN (2007) Phosphorus. In: Novais RF, Alvarez UVH, Barros NF, Fontes RLF, Cantarutti RB, Neves JCL (eds) Soil Fertility: 1. Brazilian Society of Soil Science, Viҫosa, M.G, pp 471–550 (in Portuguese)
Powell N, Shilton A, Chisti Y, Pratt S (2009) Towards a luxury uptake process via microalgae-defining the polyphosphate dynamics. Water Res. https://doi.org/10.1016/j.watres.2009.06.011
Rao DLN, Burns RG (1991) The effect of surface growth on blue-green algae and bryophytes on some microbiological, biochemical and physical soil properties. Biol Fert Soils 9:239–244
Ray K, Mukherjee C, Ghosh AN (2013) A way to cure phosphorus toxicity in the environment: use of polyphosphate reservoir of cyanobacteria and microalga as a safe alternative phosphorus biofertilizer for Indian Agriculture. Environ Sci Technol 47:11378–11379. https://doi.org/10.1021/es403057c
Renuka N, Prasanna R, Sood A, Ahluwalia AS, Bansal R, Babu S, Singh R, Shivay YS, Nain L (2016) Exploring the efficacy of wastewater-grown microbial biomass as a biofertilizer for wheat. Environ Sci Pollut Res 23:6608–6620
Rogers SL, Burn RG (1994) Changes in aggregate stability, nutrient status, indigenous microbial populations, and seedling emergence, following inoculation of soil with Nostoc muscorum. Biol Fert Soils 18:209–215
Roychoudhury P, Kaushik BD (1989) Solubilization of Mussoorie rock phosphate by cyanobacteria. Curr Sci 58:569–570
Roychoudhury P, Kaushik BD, Krishnamurthy GSR, Venkataraman GS (1979) Effect of blue-green algae and Azolla application on the aggregation status of the soil. Curr Sci 48:454–455
Saha KC, Mandal LN (1979) Effect of algal growth on the availability of phosphorus, iron and manganese in rice soil. Plant Soil 52:139–146
Santos FM, Pires JCM (2018). Nutrient recovery from wastewaters by microalgae and its potential application as bio-char, Bioresource. Technol., https://doi.org/10.1016/j.biotech.2018.07.19.
Schreiber C, Schiedung H, Harrison L, Briese C, Ackermann B, Kant J, Schrey SD, Hofmann D, Singh D, Ebenhöh O, Amelung W, Schurr U, Mettler-Altmann T, Huber G, Jablonowski ND, Nedbal L (2018) Evaluating potential of green alga Chlorella vulgaris to accumulate phosphorus and to fertilize nutrient poor soil substrates for crop plants. J Appl Phycol. https://doi.org/10.1007/s10811-018-1390-9.
Sigurnjak I, Vaneeckhaute C, Michels E, Ryckaert B, Ghekiere G, Tack FMG, Meers E (2017) Fertilizer performance of liquid fraction of digestate as synthetic nitrogen substitute in silage maize cultivation for three consecutive years. Sci Total Environ 599:1885–1894
Singh AL, Singh PL (1989) Nitrogen Fixation in Indian Rice Fields (Azolla and Blue-green Algae). Agro-Botanical Publishers, Bikaner, India
Singh RN (2008) Reclamation of Usar Lands. “Role of Blue-green Algae in Nitrogen Economy of Indian agriculture”. Indian Council of Agricultural Research, New Delhi, 1961.9: 707–718, https://doi.org/10.1007/s00253008-1518-y.
Sole-Bundó M, Cucina M, Tapias J, Gigliotti G, Garfi M, Ferrer I (2017) Assessing the agricultural reuse of the digestate from microalgae anaerobic digestion and co-digestion with sewage sludge. Sci Total Environ 586:1–9
Solovchenko A, Verschoor AM, Jablonowski ND, Nedbal L (2016) Phosphorus from wastewater to crops: an alternative path involving microalgae. Biotechnol Adv 34:550–564
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96. https://doi.org/10.1263/jbb.101.87
Stirk WA, Van Staden J (2010) Flow of cytokinins through the environment. Plant Growth Regul 62:101–116
Subhashini D, Kaushik BD (1981) Amelioration of sodic soils with blue-green algae. Aust J Soil Res 19:361–366
Vaishampayan A (1998) Physiological responses of genetically improved nitrogen-fixing cyanobacteria to agrochemicalization in relation to paddy culture: prospects as a source material for engineering herbicide sensitivity and resistance in plants. In: Hemantaranjan A (ed) Advances in Plant Physiology, vol I. Scientific Publishers. Jodhpur, India, pp 191–220
Vaishampayan A, Sinha RP, Hader DP, Dey T, Gupta AK (2001) Cyanobacterial biofertilizers in rice agriculture. Bot Rev 67:453–516
Venkataraman GS (1993) Blue-green algae (cyanobacteria). In: Tata SN, Wadhwani AM, Mehdi MS (eds) Biological Nitrogen Fixation. Indian Council of Agricultural Research, New Delhi, pp 45–76.
Wang B, Li Y, Wu N, Lan CQ (2008) CO2 bio-mitigation using microalgae. Appl Microbiol Biotechnol 79:707–718. https://doi.org/10.1007/s00253-008-1518-y
Watanabe I, Cholikul W (1979) Field studies on nitrogen fixation in paddy soil, in: Nitrogen and Rice Symposium Proceedings, IRRI, Philippines, pp 223–239.
Whitton BA (2000) Soils and rice-fields. In: Whitton BA, Potts M (eds) The Ecology of Cyanobacteria: Their Diversity in Time and Space. Springer, Netherland, pp 233–255
Yu S, Feng Y, Xue L, Sun H, Han L, Yang L, Sun Q, Chu Q (2019) Biowaste to treasure: application of microbial-aged hydrochar in rice paddy could improve nitrogen use efficiency and rice grain free amino acids. J Clean Prod 240:118–180. https://doi.org/10.1016/j.clepro.2019.118180
Yuan H, Ge T, Chen C, O’Donnell AG, Wu J (2012) Significant role for microbial autotrophy in the sequestration of soil carbon. Appl Environ Microbiol 78:2328–2336
Zhu S, Wang Y, Jen Xu, Shang C, Wang Z, Xu J, Yuan Z (2015) Luxury uptake of phosphorus changes the accumulation of starch and lipid in Chlorella sp. under nitrogen depletion. Bioresour Technol 198:165–171. https://doi.org/10.1016/j.biotech.2015.08.142
Funding
Open access funding provided by Széchenyi István University (SZE).
Author information
Authors and Affiliations
Contributions
LM was involved in the compilation of literature and draft into a review paper; TJ participated in supervision and incorporation of scientific views; ÖV aided in supervision. ZM was involved in supervision and rectification of the review paper. The manuscript has been read, reviewed, and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mutum, L., Janda, T., Ördög, V. et al. Biologia Futura: potential of different forms of microalgae for soil improvement. BIOLOGIA FUTURA 73, 1–8 (2022). https://doi.org/10.1007/s42977-021-00103-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42977-021-00103-2