Abstract
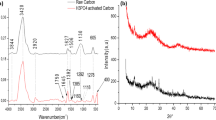
This study evaluated how acid treatment affects the ability of customized beads of activated carbon (BAC) to remove formaldehyde from air. Two different acids (hydrofluoric acid and sulfuric acid) were used to modify the surface of BAC prepared from a polymer material. The acid-modified BACs were further subjected to heat treatment. Physical and chemical characteristics of modified and unmodified BACs were investigated using nitrogen adsorption, Fourier transform infrared spectroscopy, Raman spectroscopy, X-ray fluorescence, and X-ray photoelectron spectroscopy. Formaldehyde removal was evaluated under both dry and moist conditions. From the results, acid treatment clearly improved the adsorption performance, especially under the moist condition. Qualitative and quantitative surface analyses were conducted, mainly to examine the amount of O-bonds after acid treatment and the formation of S–O or Cl–O on BAC.










Similar content being viewed by others
Data availability statement
The Data used to support the findings of this study are included within the article.
References
Duong A, Steinmaus C, McHale CM, Vaughan CP, Zhang L (2011) Reproductive and developmental toxicity of formaldehyde: a systematic review. Mutat Res 728(3):118–138
Andersen ME, Gentry PR, Swenberg JA, Mundt KA, White KW, Thompson C, Bus J, Sherman JH, Greim H, Bolt H, Marsh GM, Chechkoway H, Coggon D, Clewell HJ III (2019) Considerations for refining the risk assessment process for formaldehyde: results from an interdisciplinary workshop. Regul Toxicol Pharmacol 106:210–223
Blair A, Saracci R, Stewart PA, Hayes RB, Shy C (1990) Epidemiologic evidence on the relationship between formaldehyde exposure and cancer. Scand J Work Environ Health 16(3):381–393
Lee CY, Chiang CM, Wang YH, Ma RH (2007) A self-heating gas sensor with integrated NiO thin-film for formaldehyde detection. Sens Actuators, B Chem 122(2):503–510
Gilbert NL, Guay M, Gauvin D, Dietz RN, Chan CC, Lévesque B (2008) Air change rate and concentration of formaldehyde in residential indoor air. Atmos Environ 42(10):2424–2428
Costa S, Costa C, Madureira J, Valdiglesias V, Teixeira-Gomes A, de Pinho PG, Laffon B, Teixeira JP (2019) Occupational exposure to formaldehyde and early biomarkers of cancer risk, immunotoxicity and susceptibility. Environ Res 179:108740
Leso V, Macrini MC, Russo F, Iavicoli I (2022) Formaldehyde exposure and epigenetic effects: a systematic review. Appl Sci 10(7):2319
Kulle TJ, Sauder LR, Hebel JR, Green DJ, Chatham MD (1987) Formaldehyde dose-response in healthy nonsmokers. Japca. 37(8):919–924
Castro-Hurtado I, Mandayo GG, Castaño E (2011) Thickness influence on gas sensing characteristics of NiO thin films for formaldehyde detection. In: Proceedings of the 8th Spanish Conference on Electron Devices CDE’. pp 1–4
Wan Y, Fan X, Zhu T (2011) Removal of low-concentration formaldehyde in air by DC corona discharge plasma. Chem Eng J 171(1):314–319
Li C, Shen Y, Jia M, Sheng S, Adebajo MO, Zhu H (2008) Catalytic combustion of formaldehyde on gold/iron-oxide catalysts. Catal Commun 9(3):355–361
Chen D, Qu Z, Shen S, Li X, Shi Y, Wang Y, Fu Q, Wu J (2011) Comparative studies of silver based catalysts supported on different supports for the oxidation of formaldehyde. Catal Today 175(1):338–345
Quiroz Torres J, Royer S, Bellat JP, Giraudon JM, Lamonier JF (2013) Formaldehyde: catalytic oxidation as a promising soft way of elimination. Chemsuschem 6(4):578–592
Ryu DY, Kim DW, Kang YJ, Lee Y, Nakabayashi K, Miyawaki J, Park JI, Yoon SH (2022) Preparation of environmental-friendly N-rich chitin-derived activated carbon for the removal of formaldehyde. Carbon Lett. 32:1–7
Lee H, Park RS, Lee HW, Hong Y, Lee Y, Park SH, Jung SC, Yoo KS, Jeon JK, Park YK (2016) Adsorptive removal of atmospheric pollutants over Pyropia tenera chars. Carbon letters 19:79–88
Moon HS, Kim IS, Kang SJ, Ryu SK (2014) Adsorption of volatile organic compounds using activated carbon fiber filter in the automobiles. Carbon letters 15(3):203–209
Kang YJ, Jo HK, Jang MH, Ma X, Jeon Y, Oh K, Park JI (2022) A brief review of formaldehyde removal through activated carbon adsorption. Appl Sci 12(10):5025
Choi SS, Lee JH, Jin YM, Lee SH (2019) Adsorption characteristics of volatile organic compounds onto lyocell-based activated carbon fibers. Carbon Letters 29(6):633–642
Azhagapillai P, Al Shoaibi A, Chandrasekar S (2021) Surface functionalization methodologies on activated carbons and their benzene adsorption. Carbon Letters 31(3):419–426
Zhang L, Peng Y, Zhang J, Chen L, Meng X, Xiao FS (2016) Adsorptive and catalytic properties in the removal of volatile organic compounds over zeolite-based materials. Chin J Catal 37(6):800–809
Agarwal M, Dave M, Upadhayaya S (2011) Adsorption of formaldehyde on treated activated carbon and activated alumina. Curr World Environ 6(1):53–59
Srisuda S, Virote B (2008) Adsorption of formaldehyde vapor by amine-functionalized mesoporous silica materials. J Environ Sci 20(3):379–384
Son BC, Park CH, Kim CS (2020) Fabrication of antimicrobial nanofiber air filter using activated carbon and cinnamon essential oil. J Nanosci Nanotechnol 20(7):4376–4380
Wee JH, Bae Y, Ahn H, Choi YO, Jeong E, Yeo SY (2022) Fibrous and granular activated carbon mixed media for effective gas removal as a cabin air filter. Carbon Lett. 32:1–8
Liu M, Xiao C (2018) Research progress on modification of activated carbon. E3S Web Conf. 38:02005
de Falco G, Li W, Cimino S, Bandosz TJ (2018) Role of sulfur and nitrogen surface groups in adsorption of formaldehyde on nanoporous carbons. Carbon 138:283–291
An HB, Yu MJ, Kim JM, Jin M, Jeon JK, Park SH, Kim SS, Park YK (2012) Indoor formaldehyde removal over CMK-3. Nanoscale Res Lett 7(1):1–6
Zhou P, Zhu X, Yu J, Xiao W (2013) Effects of adsorbed F, OH, and Cl ions on formaldehyde adsorption performance and mechanism of anatase TiO2 nanosheets with exposed 001 facets. ACS Appl Mater Interfaces 5(16):8165–8172
Caturla F, Molina-Sabio M, Rodriguez-Reinoso F (1991) Preparation of activated carbon by chemical activation with ZnCl2. Carbon 29(7):999–1007
Lee KJ, Miyawaki J, Shiratori N, Yoon SH, Jang J (2013) Toward an effective adsorbent for polar pollutants: formaldehyde adsorption by activated carbon. J Hazard Mater 260:82–88
Netala VR, Kotakadi VS, Nagam V, Bobbu P, Ghosh SB, Tartte V (2015) First report of biomimetic synthesis of silver nanoparticles using aqueous callus extract of Centella asiatica and their antimicrobial activity. Appl Nanosci 5(7):801–807
Karnati SR, Høgsaa B, Zhang L, Fini EH (2020) Developing carbon nanoparticles with tunable morphology and surface chemistry for use in construction. Constr Build Mater 262:120780
Masoudian N, Rajabi M, Ghaedi M (2019) Titanium oxide nanoparticles loaded onto activated carbon prepared from bio-waste watermelon rind for the efficient ultrasonic-assisted adsorption of congo red and phenol red dyes from wastewaters. Polyhedron 173:114105
Juan Y, Ke-Qiang Q (2009) Preparation of activated carbon by chemical activation under vacuum. Environ Sci Technol 43(9):3385–3390
Li L, Liu S, Liu J (2011) Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J Hazard Mater 192(2):683–690
Przepiórski J (2006) Enhanced adsorption of phenol from water by ammonia-treated activated carbon. J Hazard Mater 135(1–3):453–456
Barroso-Bogeat A, Alexandre-Franco M, Fernández-González C, Gómez-Serrano V (2014) FT-IR analysis of pyrone and chromene structures in activated carbon. Energy Fuels 28(6):4096–4103
Choi CH, Park SH, Woo SI (2012) Binary and ternary doping of nitrogen, boron, and phosphorus into carbon for enhancing electrochemical oxygen reduction activity. ACS Nano 6(8):7084–7091
Ma X, Li L, Chen R, Wang C, Zhou K, Li H (2018) Porous carbon materials based on biomass for acetone adsorption: effect of surface chemistry and porous structure. Appl Surf Sci 459:657–664
Wang J, Krishna R, Yang J, Dandamudi KPR, Deng S (2015) Nitrogen-doped porous carbons for highly selective CO2 capture from flue gases and natural gas upgrading. Mater Today Commun 4:156–165
Sevilla M, Fuertes AB (2006) Catalytic graphitization of templated mesoporous carbons. Carbon 44(3):468–474
Sevilla M, Fuertes AB (2013) Fabrication of porous carbon monoliths with a graphitic framework. Carbon 56:155–166
Guo Y, Zeng Z, Zhu Y, Huang Z, Cui Y, Yang J (2018) Catalytic oxidation of aqueous organic contaminants by persulfate activated with sulfur-doped hierarchically porous carbon derived from thiophene. Appl Catal B 220:635–644
Niki H, Maker PD, Breitenbach LP, Savage CM (1978) FTIR studies of the kinetics and mechanism for the reaction of Cl atom with formaldehyde. Chem Phys Lett 57(4):596–599
Seredych M, Łoś S, Giannakoudakis DA, Rodríguez-Castellón E, Bandosz TJ (2016) Photoactivity of g-C3N4/S-doped porous carbon composite: synergistic effect of composite formation. Chemsuschem 9(8):795–799
Xu Z, Tian D, Sun Z, Zhang D, Zhou Y, Chen W, Deng H (2019) Highly porous activated carbon synthesized by pyrolysis of polyester fabric wastes with different iron salts: pore development and adsorption behavior. Colloids Surf, A 565:180–187
Hu P, Duan Y, Ding W, Zhang J, Bai L, Li N, Wei H (2017) Enhancement of mercury removal efficiency by activated carbon treated with nonthermal plasma in different atmospheres. Energy Fuels 31(12):13852–13858
Tolbert MA, Pfaff J, Jayaweera I, Prather MJ (1993) Uptake of formaldehyde by sulfuric acid solutions: impact on stratospheric ozone. J Geophys Res Atmos 98(D2):2957–2962
Kwon DW, Seo PW, Kim GJ, Hong SC (2015) Characteristics of the HCHO oxidation reaction over Pt/TiO2 catalysts at room temperature: the effect of relative humidity on catalytic activity. Appl Catal B 163:436–443
Wang Z, Pei J, Zhang JS (2012) Modeling and simulation of an activated carbon-based botanical air filtration system for improving indoor air quality. Build Environ 54:109–115
Acknowledgement
This work was supported by Korea Environment Industry & Technology Institute (KEITI) through the Prospective Green Technology Innovation Project, funded by Korea Ministry of Environment (MOE) (2020003160004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
TThe authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, YJ., Jo, HK., Jang, MH. et al. Acid treatment enhances performance of beads activated carbon for formaldehyde removal. Carbon Lett. 33, 397–408 (2023). https://doi.org/10.1007/s42823-022-00428-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-022-00428-5