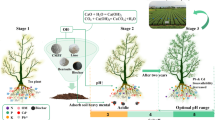
Abstract
Long-term consumption of tea with high fluoride (F) content has a potential threat to human health. The application of different amounts of biochar to reduce F accumulation in tea leaves has been little studied. In this study, a pot experiment was conducted to investigate the effect of biochar amounts (0, 0.5%, 2.5%, 5.0%, 8.0%, and 10.0%, w/w) on tea F content during the tea plant growth. Changes in tea quality, soil F fraction, and soil properties caused by biochar and the relationship with tea F accumulation were also considered. The results showed that the application of biochar amendment significantly reduced water-soluble F contents in tea leaves compared to CK (without biochar), especially in the 8.0% treatment (72.55%). Overall, biochar contributed to improving tea polyphenols and caffeine, but had no significant impact on free amino acids and water leachate. Compared with CK, 5.0–10.0% biochar significantly increased soil water-soluble F content due to the substitution of F− with OH− under high pH. Additionally, biochar applied to tea garden soil was effective in decreasing the soil exchangeable aluminum (Ex-Al) content (46.37–91.90%) and increasing the soil exchangeable calcium (Ca2+) content (12.02–129.74%) compared to CK, and correlation analysis showed that this may help reduce F enrichment of tea leaves. In general, the application of 5.0–8.0% biochar can be suggested as an optimal application dose to decrease tea F contents while simultaneously improving tea quality.
Graphical Abstract

Highlights
-
Biochar application significantly reduced tea F accumulation varied with amounts.
-
Biochar improved nutrients uptake, which was beneficial for tea quality improvement.
-
The change in caused by biochar played an essential role in reducing tea F.
Similar content being viewed by others
1 Introduction
Fluoride (F) is a widely reported pollutant that enters soil and water through mining, coal burning, smelting, fluoridated fertilizers and pesticides (Wang et al. 2019; Rizzu et al. 2021). Tea (Camellia sinensis), one of the most economically important crops in China, is recognized as a hyperaccumulator of F (Luo et al. 2021; Peng et al. 2021). Most of the F absorbed by tea plants from the soil accumulates in the leaves and can be as high as 2000 mg g-1in mature or fallen leaves (Yi et al. 2017; Fung et al. 1999). Tea, obtained from the leaves of tea plants, is widely consumed as a non-alcoholic beverage worldwide and is also a significant source of dietary F (Satou et al. 2021; Lin et al. 2022). When brewing, approximately 18.0–99.0% of F in tea leaves is released into the tea broth (Koblar et al. 2012; Lv et al. 2013; Das et al. 2017). Drinking tea with a normal concentration of F is considered somewhat to be safe and even beneficial to health (Guo et al. 2020). However, long-term high consumption of tea made from older leaves (e.g., dark tea) could lead to tea-drinking fluorosis, such as dental fluorosis and bone fluorosis, which has been identified as one of the endemic species of fluorosis in the western minority areas of China (Zhang et al. 2019; Kabir et al. 2020; Chu et al. 2021). Additionally, too much tea F content could affect the tea plant's growth and the taste and aroma of tea leaves (Yang et al. 2015; Cai et al. 2016). Therefore, it is very urgent to control tea F content strictly. However, the current efforts to reduce F in tea mainly depend on tea processing, and an effective means to prevent the accumulation of F during the growth of tea plants is lacking. Thus, F control and reduction is necessary for tea gardens. Exploring effective measures to prevent the F accumulation in tea raw materials is of great importance for reducing the potential human health risks.
At present, many technologies such as biosorption, chemical stabilization, soil washing, and electrokinetic remediation have been used to remove F from the environment (Zhou et al. 2014; Moon et al. 2015; Natarajan and Al Qasmi 2018; Wang et al. 2022a). Several studies have reported that adding exogenous substances e.g. CaO, dolomite, lime, peat, KCl, and humate to tea garden soil seems to be a more sustainable and effective immobilisation strategy to reduce F uptake by tea plants (Ruan et al. 2004; Yi et al. 2017; Huang et al. 2020). Biochar, as a carbon-rich amendment material produced by pyrolysis of agricultural wastes under limited oxygen conditions, is widely used in soil pollution remediation, promoting carbon sequestration, and enhancing crop yield because of its high carbon content, large specific surface area, and rich functional groups (Hossain et al. 2020; Wang et al. 2022c; Yan et al. 2022b; Yao et al. 2022). Kumar et al. (2022) reviewed the promising potential of biochar materials for the treatment of fluoride-contaminated groundwater and industrial wastewater. Fan et al. (2022) and Zhu et al. (2016) found that biochar reduced the mobility of F in soil and had a significant fixation effect. Ghassemi-Golezani and Farhangi-Abriz (2019) reported that biochar helps to reduce the F solubility in fluoride-contaminated soil thereby reducing the F content in safflower tissues. However, to date, only one study has examined biochar as a soil amendment to reduce F content in tea leaves (Gao et al. 2012). In addition, biochar is increasingly being used to improve tea garden soil properties to meet the growth needs of tea plants, thus improving tea quality (Wang et al. 2014; Yan et al. 2021; Yi et al. 2022). All these aspects suggest that a better knowledge on the effects of biochar on tea F accumulation and tea quality is critical for the sustainable development of tea garden ecosystems.
The remediation performance of biochar in soil and plants is not only influenced by the feedstock and preparing conditions, but also depends to a large extent on the application rates (Lv et al. 2021; Nobaharan et al. 2022; Yan et al. 2022b). However, there are contradictory reports on the fixation of soil available F (water-soluble state F, Ws-F) at different biochar amounts. Sun et al. (2017) found that the adsorption amount and rate of Ws-F in tea garden soil decreased gradually with the increase of biochar addition (0.25–2.0%, w/w). However, other studies have shown that biochar amendment dosage from 4.0% to 12.0% gradually enhanced the adsorption of Ws-F in F-contaminated soil (Fan et al. 2022). It is well known that tea plant F mainly depends on soil F bioavailability (Sun et al. 2022). Unfortunately, none of these studies above examined whether biochar would affect the accumulation of F in tea leaves. This means that the effect of biochar amounts on F uptake and accumulation in the soil-tea system is a complex process that lacks comprehensive understanding. Gao et al. (2012) only explored the effect of 2.5% and 5.0% biochar on the accumulation of F in tea leaves, and the effect of higher amount is unknown. Therefore, it is necessary to expand the dose range of biochar application to verify its reduction effect on tea F and explore the potential mechanism. The physicochemical properties of biochar are the main determinant of their ability to change the physicochemical properties of soil (Shi et al. 2020). Previous studies have shown that biochar mainly influences soil pH, exchangeable aluminum (Ex-Al), and the presence of soil exchangeable base cations, all of which affect soil F enrichment by tea leaves (Fung et al. 1999; Ruan et al. 2004; Wang et al. 2021). Based on the above analysis, we hypothesized that the more the amount of biochar applied, the better the effect of tea F reduction.
Tea is a typical acid-loving crop, which can absorb and accumulate large amounts of soil Al (Jiang et al. 2022; Yang et al. 2022). Previous studies have shown that soil Ws-F will chelate with soil Al3+ to form F-Al complexes, which will be absorbed by the roots of tea plants and then transported to the leaves (Ruan et al. 2003; Zhang et al. 2012, 2015). Increasing the supply of exogenous Al can promote the accumulation of F in tea leaves (Zhang et al. 2015). Long et al. (2021) showed that urea causes an increase in the dissolved F and Al content in tea garden soil and migrates to tea as F-Al complexes, and the key to reducing F content in tea is to reduce the F–Al complex content in the soil. Furthermore, it has also been reported that F and Al are present in tea in the form of ligand compounds (e.g., AlF2+, AlF2+, and AlFx(3−x)−), which may help to weaken the toxicity of F (Ruan et al. 2003; Xie et al. 2007; Cai et al. 2014). Based on the above studies, it can be inferred that the presence of Al in the soil plays a key role in the enrichment of F in tea leaves. In recent years, excessive applications of nitrogen (N) fertilizers and large tea harvests have increased the acidification rate of tea garden soil (Li et al. 2016; Yang et al. 2018). As soil pH decreases significantly, soil exchangeable Al (Ex-Al) becomes more soluble and effective, and high levels of Al enrichment adversely affect the growth and quality of tea plants (Yan et al. 2018, 2020). Many studies have reported that biochar can increase soil pH to effectively alleviate soil Al toxicity (especially Ex-Al) and is an effective way to avoid soil acidification in tea gardens (Wang et al. 2018; Shi et al. 2020; Li et al. 2021; Dong et al. 2022). In conclusion, although the presence of Al in soil has been reported to affect the transport and enrichment of tea F, this aspect has not been sufficiently studied so far, let alone under biochar application conditions. Whether the effect of biochar on Ex-Al in tea garden soil will reduce the formation of F-Al complexes to reduce tea F accumulation has not been investigated and still largely unknown.
The aims of the present work were: (i) to study the effects of different biochar treatments on tea F accumulation, as well as on tea quality; (ii) to explore the effects on soil properties, e.g., pH, exchangeable base cations, and soil Ex-Al; and (iii) to investigate the potential mechanism of biochar treatments to reduce F accumulation in tea leaves. The obtained information helps to understand the usage of biochar in the tea gardens, which is of good application to realize the healthy and sustainable development of the tea garden ecosystem in southern China.
2 Materials and methods
2.1 Soil and biochar characteristics
Acid yellow soil samples (0–20 cm) were collected in a tea garden (111°53′E, 28°51′N) in Yiyang city, Hunan Province, China. After air-drying and removing plant debris and stones, the soil samples were passed through a 2.0 mm sieve for pot experiments. Soil pH was 5.30, organic carbon was 13.48 g kg−1, total nitrogen (TN) was 1.46 g kg−1, total phosphorus (TP) was 0.48 g kg−1, total potassium (TK) was 16.36 g kg−1, soil total F (T-F) content was 553.74 mg kg−1, and soil Ws-F was 2.90 mg kg−1. Biochar was produced by slow pyrolysis of miscellaneous wood materials at 500 ℃ under anoxic conditions for 2–3 h and provided by Henan Lize Environmental Protection Technology Co., Ltd (Zhengzhou, China) (Yan et al. 2022a). Biochar characterization included pH in H2O (8.86), specific surface area of 26.03 m2 g−1, particle size of 35.46 um, total pore volume of 0.019 cm3 g−1, T-F content of 229.96 mg kg−1, and Ws-F of 2.67 mg kg−1. Other basic properties of the studied soil and biochar are listed in Additional file 1: Table S1.
2.2 Pot experiment
In general soil amendment processes, although biochar at rates within 5.0% is often used to stabilize contaminants in contaminated soils, high application rates (5.0% or above) are usually required to attain significant effect (Ali et al. 2020; Jun et al. 2020; Azeem et al. 2021). In particular, 10.0% biochar is also frequently used to reduce the mobility of contaminants in the soil, as shown in Additional file 1: Table S2. Therefore, in this study, biochar was repeatedly mixed with 7.0 kg dry soil in plastic pots (25.0 cm high, 18.0 cm in bottom diameter, 23.0 cm in top diameter) to prepare 6 treatments at doses of 0, 0.5%, 2.5%, 5.0%, 8.0%, and 10.0% (mass ratio of biochar/soil, w/w), labelled as CK, B1, B2, B3, B4 and B5, respectively. Three 1-year-old tea seedlings (Fudingdabai, 35.0 cm high) from the Tea Research Institute of Hunan Province, Changsha, China were transplanted into each pot. Each of the six treatments was replicated three times, with each replicate consisting of 3 pots to ensure sampling volume, and the experiment had a total of 54 pots. The tea plants were cultivated in a solar greenhouse and watered regularly to maintain soil moisture at 75.0% of field capacity during the growth period. There was no topdressing and manual weeding, and insect removal during the experiment.
2.3 Plant sample analysis
Tea seedlings were transplanted on January 18, 2021, and tea leaves were picked on October 24, 2021 to ensure that tea leaves have enough growing period. The 4–6th mature leaves were picked from each treatment (9 pots), and mixed randomly in every 3 pots with a total weight of not less than 300.0 g per treatment. Tea samples collected would be put into polyethene plastic bags and recorded with label paper. After picking, the tea seedlings were carefully uprooted and treated with deionized water. The roots, stems, and leaves were separated and put into clean envelopes for green processing. The tea was dried at 60 ℃ to constant weight, and the tea Ws-F test method is in line with national standards (GB 19965-2005, China standard). The T-F in tea plant tissues was referred to the method of Gan et al. (2021) by the alkali fusion method. The contents of Ws-F and T-F were determined by the F electrode method (PF-202-L, Leici, China). The water-soluble Al (Ws-Al) contents in tea plant tissues were extracted using the above-described Ws-F method, and determined using ICP-OES (ICPE-9000, Shimadzu, Japan). The tea polyphenols (TPP) were determined by (GB/T 8313-2018), free amino acids (FAA) by (GB/T 8314-2013), caffeine (CAF) by (GB/T 8312-2013), and tea water leachate (WLS) by (GB/T 8305-2013).
2.4 Soil samples analysis
After tea harvest, pot topsoil (0-15 cm) was collected for air drying and grinding. The soil F fractions can be classified as water-soluble F (WS-F), exchange F (Ex-F), organically bound F (OM-F), Fe/Mn oxides-bound F (Fe/Mn-F), and residue (Res-F). The soil F fraction sequential extraction procedure was referred to Gao et al. (2012) and Gan et al. (2021), and the content was determined by the F electrode method. Briefly, soil Ftotal was extracted by the alkali fusion method, Ws-F was extracted with 70 ℃ hot water; Ex-F was extracted with 1 M MgCl2; OM-F was extracted with 0.04 M NH2OH·HCl in 20.0% CH3COOH; Fe/Mn-F was extracted with 0.02 M HNO3, 30.0% H2O2, 3.2 M NH4AC, and Res-Fwas extracted by Ftotal minus other F fractions, with GBW07939 as the standard control. Soil exchangeable Al (Ex-Al), extracted with 1 M KCl, was measured using ICP-OES (Ruan et al. 2006). Soil pH was measured at a 1:2.5 ratio of solid-to-liquid. Soil exchangeable base cations were analyzed according to NY/T 295–1995.
2.5 Data and statistical analysis
Data were processed in SPSS package (version 25.0) and expressed as means ± standard deviation (SD) or means of three replicates. Statistical differences between control and treatment groups were performed using the ANOVA method, followed by the LSD tests (p < 0.05). Pearson's correlation analysis was conducted to determine the tea F content associations with soil-tea system parameters. The figures in this study were drawn by Origin 2020b.
3 Results
3.1 Effects of biochar on tea F accumulation
This study measured two types of tea plant F contents based on the laboratory conditions, including Ws-F and T-F. As shown in Fig. 1a, the Ws-F content of tea leaves in the biochar treatment group was significantly lower compared with CK during the tea plant growth period, and the inhibition ability of different amounts of biochar on Ws-F accumulation was different. The WS-F of tea leaves in the control group was 715.92 mg kg−1, occupying 48.8% of tea T-F (Additional file 1: Fig. S1). Notably, the biochar-treated tea also exhibited low T-F accumulation and decreased significantly with increasing doses ( p < 0.05), which was consistent with the trend of Ws-F in tea leaves. The accumulated Ws-F was mainly concentrated in tea leaves, which accounted for 94.60% of the total accumulated Ws-F in the whole tea plants (Fig. 1a). Compared with CK, biochar reduced the tea WS-F by 27.74%, 48.93%, 69.60%, 72.55%, and 51.47%, respectively. From this result, it can be inferred that the greater the amount of biochar, the smaller the change in WS-F content of tea leaves: B1 (27.74%) > B2 (21.19%) > B3 (20.67%) > B4 (2.95%) > B5 (-21.08%). In addition, the decrease in tea Ws-F was not proportional to the increase in the amount of biochar, and the 8.0% treatment had the lowest , 196.54 mg kg−1. However, there was no significant difference in WS-F accumulated between 2.5% and 10.0% biochar application rates (p < 0.05) by continuing to increase the amount of biochar. It can be seen that our initial hypothesis was only partially met, indicating that there was a limit to the F reduction by biochar, and 8.0% reached the abundance value.
In this study, adding biochar to tested soil did not show a significant effect on the Ws-F content of tea roots compared with the control (Fig. 1a). We observed that biochar treatments in pot soils significantly decreased the Ws-F uptake by tea stems. The T-F contents in tea stems significantly decreased, and the changes in roots were not significant (p < 0.05) at different biochar application rates compared to CK (Additional file 1: Fig. S1). It is worth mentioning that 5.0% biochar showed a better inhibition effect on Ws-F enrichment of tea stems, but the migration of Ws-F from roots to stems increased with the further increase of biochar content. The 8.0% biochar treatment had the lowest T-F content in stems, and that in root was the lowest at 0.5%, which were 39.4% and 15.6% lower than CK, respectively.
Tea plant can take in both F and Al, and the decrease of F content in tea by biochar was closely related to the effect of biochar on Al in tea (Ruan and Wong 2001; Gao et al. 2014). The distribution of Ws-Al contents in tea leaves, stems, and roots under different biochar treatments is listed in Fig. 1b. The Ws-Al contents of tea leaves, stems, and roots in the control soil were significantly higher than the Ws-F contents of the same parts (p < 0.05). The accumulation of Ws-Al contents in tea plant tissues in all treatments was leaves > roots > stems. In general, high biochar amounts (8.0–10.0%) were significantly more effective in inhibiting Ws-Al in tea stems and roots than low amounts (0.5–2.5%). Except for the 10.0% biochar treatment, the Ws-Al of tea leaves, stems, and roots significantly decreased with increasing application rates (p < 0.05). Ws-Al of tea roots and stems reached the lowest value at 8.0% addition, while tea leaves at 5.0% addition, with 47.43%, 45.14%, and 88.12% reduction compared to CK. Additionally, the highest application rate (10.0%) of biochar again increased tea Ws-Al content compared with 8.0% treatment, which was also observed in tea Ws-F.
3.2 Effects of biochar on tea quality
Biochar treatments had significant effects on tea quality (p < 0.05) (Fig. 2). The results of the study revealed that the contents of TPP fluctuated between 14.24% and 18.31% in all treatments. Compared with untreated soil, 2.5–10.0% biochar significantly increased the contents of TPP by 17.6%, 19.0%, 18.0%, and 9.1% (p < 0.05), respectively (Fig. 2a). Moreover, adding 0.5% biochar significantly increased the FAA content compared to CK, and a higher biochar content caused the decrease of FAA content, although there was no significant difference (p < 0.05) (Fig. 2b). Compared to untreated soil, the highest FAA content was observed in the 0.5% biochar treatment (2.76%), followed by 2.5% biochar (2.41%).
The results showed that the WLS and TPP contents showed a similar trend as influenced by the biochar amount, e.g., 0.5% treatment exhibited the lowest value and maximum content in 5.0% biochar-treated soil (Fig. 2c). Interestingly, the WLS content of the control treatment was the lowest, and none of the biochar treatments differed from CK to a significant level. Compared with the control, the application of biochar increased the content of CAF in tea (Fig. 2d). There was no significant difference in the effect of 0.5% and 2.5% biochar (p < 0.05). The highest content of CAF was 1.77% in 5.0% biochar treatment soil, which was twice as much as that of CK, but the CAF content in the leaves decreased as the amount of biochar applied in the soil increased. In general, in this study, we found that 5.0–8.0% biochar has a greater advantage for TPP, WLS, and CAF enhancement than lower amounts of biochar (0.5–2.5%).
3.3 Effects of biochar on soil F fractions and soil properties
In this current study, soil F was classified into five fractions using a sequential chemical extraction method (Table 1). The changes in F fraction contents varied among different amounts of biochar treatments. No significant differences in soil Ftotal and Res-F among the six treatments were observed on the basis of ANOVA (p < 0.05). The soil Ws-F content decreased and then increased, whereas an opposite trend was observed for the soil Fe/Mn-F. The changes in soil Ex-F and OM-F were irregular. Results showed soil Ws-F content among the biochar treatments in all tested soils ranged from 2.25 to 4.37 mg kg−1. Soil Ws-F contents were not affected by the lower biochar application rate (0.5–2.5%) when compared to CK. However, it could be clearly seen that the application of 5.0–10.0% biochar significantly increased the soil Ws-F content by 24.8%, 63.8%, and 72.0%, respectively (p < 0.05). Overall, the difference in soil Ws-F content was greatest between 2.5% and 5.0% biochar. Compared with the control, the high application rate of biochar (8.0–10.0%) had a greater impact on Fe/Mn-F, while the difference was not significant at 0.5% application rate.
Soil properties, including soil pH, exchangeable base cations, and Ex-Al under different treatments, are shown in Table 2. Different amounts of biochar were significant for the soil pH. The 0.5% and 2.5% treatments slightly decreased pH compared with the control (p < 0.05). Conversely, the 5.0%, 8.0%, and 10.0% treatments significantly increased soil pH by 0.81, 1.37, and 1.63 units, respectively (p < 0.05). Compared with other treatments, the difference in soil pH was more evident under 5.0% and 8.0% biochar treatments.
Changes in exchangeable base cations were measured at the end of the pot experiment. Results showed that exchangeable Ca2+ in untreated soil was the main component of exchangeable base cations, accounting for 80.5%. The exchangeable Na+ contents were higher in all treatments than in the control treatment, but statistically insignificant (p < 0.05). As seen in Table 2, the data about exchangeable K+, Ca2+, and Mg2+ were not affected by lower biochar application rates (0.05–2.5%) compared to the unamended soil. The exchangeable K+, Ca2+, and Mg2+ significantly increased (p < 0.05) only at the high biochar rates (5.0–10.0%). The 10.0% biochar treatment enhanced soil exchangeable K+, Ca2+, and Mg2+ contents, which were 3.4, 2.3, and 1.3 times higher than CK, respectively.
Compared with the increase of soil pH, soil Ex-Al content decreased with increasing biochar application rate. According to the current study, soil Ex-Al contents of the different amount treatments were B1 > CK > B2 > B3 > B4 > B5 in descending order. In comparison to CK, the application of 2.5–10.0% biochar rates significantly decreased soil Ex-Al content (p < 0.05), except for 0.5%, and the decreases were 46.37%, 88.26%, 91.50%, and 91.90% for each treatment, respectively. The soil Ex-Al content was 1.93 mg kg−1 when added at 5.0%, and the difference in the results was not statistically significant (p < 0.05) when the amount continued to increase.
3.4 Correlation analysis
Figure 3 shows the correlation analysis of F in the soil-tea system under different biochar treatments. The Ws-F contents of tea leaves were extremely significant positive correlated with the tea stems Ws-F content, the Ws-Al contents of tea roots, stems, and leaves, and the soil Ex-Al contents, and the correlation coefficient reached 0.71, 0.90, 0.91, 0.95, and 0.89, respectively (p < 0.01). Tea Ws-F contents were significantly negatively correlated with soil Ws-F and OM-F contents (p < 0.05) and highly significantly negatively correlated with soil pH, exchangeable K+, Ca2+, and Mg2+ (p < 0.01). What could be observed was that tea Ws-F contents were positively, but not significantly, correlated with the contents of tea root Ws-F, soil Fe/Mn-F and Res-F.
Soil-tea plant system fluorine correlation analysis. WF-Leaf: soluble fluorine in leaf; WF-Stem: soluble fluorine in stem; WF-Root: soluble fluorine in root; WAl-Leaf: soluble aluminum in leaf; WAl-Stem: soluble aluminum in stem; WAl-Root: soluble aluminum in root; TPP: tea polyphenol; FAA: free amino acid; WLS: water leachate; CAF: caffeine; WS-F: soil water-soluble fluorine; Ex-F: soil exchange fluorine; OM-F: soil organically bound fluorine; Fe/Mn-F: soil ferromangan-bound fluorine; Res-F: soil residual fluorine; K+, Na+, Ca2+, and Mg2+: soil exchangeable base cations; Ex-Al: soil exchangeable aluminum. * and ** show the correlations significant at the 0.05 and 0.01 levels
The TPP and CAF were highly negatively correlated with tea Ws-F (p < 0.01). Changes in tea Ws-F contents were significantly and positively correlated with FAA (p < 0.05). The change in tea Ex-Al contents was significantly negatively correlated with TPP (p < 0.05) and highly significantly negatively correlated with CAF (p < 0.01). In terms of soil parameters, the TPP was positively correlated with soil OM-F (p < 0.05), and there was a highly significant negative correlation with Ex-Al (p < 0.01). The soil Ws-F and Ex-F had a significant direct negative effect on FAA. Whereas soil Ex-Al revealed detect positive effects on FAA (p < 0.01). Meanwhile, the value of pH, K+, and Ca2+ all were negatively correlated with FAA (p < 0.01). In addition, no significant correlations (positive or negative) were observed between WLS, CAF, and soil parameters.
4 Discussion
4.1 Biochar reduced tea F accumulation
Tea plant, as F hyperaccumulator, differs from other plants in that the above-ground F content is much higher than the below-ground F content (Peng et al. 2021). Large amounts of F absorbed by roots are readily transported through the xylem to mature leaves in the form of F− and F-Al complexes without toxic symptoms (Ruan and Wong 2001; Ruan et al. 2003; Niu et al. 2020b). Similar to the results from Yang et al. (2016), the total F content in mature leaves in control soil was above 1000 mg kg−1 (Additional file 1: Fig. S1). Previous studies reported that up to 90.0–98.0% Ws-F in tea plants was distributed in the leaves (Sha and Zheng 1994; Ruan et al. 2003; Gao et al. 2012), which was consistent with this study. Compared with tea T-F, tea Ws-Fis closely related to bioavailability and therefore poses a more toxic risk to human health (Yi et al. 2017; Peng et al. 2021). Gao et al. (2012) set the highest amount of charcoal or bamboo charcoal at 5.0% and obtained the lowest Ws-F accumulation. Ghassemi-Golezani and Farhangi-Abriz (2019) found that 5.0% biochar was superior to 2.5% biochar for F reduction in safflower (Carthamus tinctorius L.) seedlings. However, researchers did not consider the effect of higher amounts of biochar on tea F levels. Our current study emphasizes the importance of different biochar amounts. In this case, the maximum amount was innovatively set at 10.0% in this study, which is twice the amount of biochar applied in tea garden in previous studies (Gao et al. 2012; Wang et al. 2018; Yan et al. 2021). Compared with previous use of humic acid aluminum and polyphenol-Ce adsorbents to reduce F accumulation in tea leaves by 74.29% and 74.75% (Zhao et al. 2015; Huang et al. 2020), the 8.0% biochar treatment in this study was the best, reducing F accumulation by 72.55%, and the 5.0% and 8.0% treatments were not significant. According to a previous report (Cao et al. 1996), the F content in tea is considered to be within the safe range when it is maintained between 100.0–300.0 mg kg−1. Therefore, 5.0–8.0% could be considered as an acceptable amount of biochar application. Interestingly, 10.0% biochar was not as effective in reducing F in tea leaves as 8.0%. This indicates that there is a threshold when the amount of biochar applied reaches a certain level at which the Ws-F content in tea leaves no longer decreases significantly, which has been shown in previous studies (Wang et al. 2022b; Yan et al. 2022b). However, it is difficult to give specific explanations of the results obtained, and further detailed investigations are required. In particular, it is not clear whether this is attributable to the bioconcentration effect of F due to biomass reduction (Lv et al. 2021). Therefore, the effect of the amount of biochar application on tea biomass needs further study. In conclusion, the appropriate amount of biochar should be considered according to the purpose of production practice to achieve the maximum benefit while ensuring low cost.
Tea leaves are the most important F-accumulating tissues of tea plants. The high F accumulation in tea leaves is mainly due to the particular absorption, transport and accumulation mechanism of tea roots (Zhang et al. 2012). Soil F fractions, especially soil Ws-F, are easily absorbed by tea roots and then translocated, metabolized, and accumulated in the tea leaves (Yin et al. 2016; Peng et al. 2021). The present study revealed that the Ws-F content of tea leaves in the control was approximately 43 times that of the roots, which is consistent with the results of previous studies by Zhu et al. (2019). Niu et al. (2020b) found that F absorbed by tea roots was mainly transported through the xylem, but also partially through the bast of tea stems. The absorption of F by tea rootswas linearly correlated with exogenous F, and active transport was the primary form of F transport in tea plants (Peng et al. 2013; Zhang et al. 2015). According to a previous report, the F content in roots and leaves reduced under the F-contaminated soil with different biochar treatments (Ghassemi-Golezani and Farhangi-Abriz 2019). However, biochar applied to soil did not show significant effect on the uptake in T-F and Ws-F in tea roots compared with the control in this paper. This contradicts the previous study by Gao et al. (2012), which found that the addition of charcoal and bamboo charcoal significantly reduced the Ws-F content of tea roots. Different experimental conditions may explain the above differences.
4.2 Biochar helps to improve tea quality
At present, as a new type of agricultural fertilizer, biochar has been reported on the growth of maize, rape, citrus, and other economic crops, but there are few reports on the impact of tea quality (Zhang et al. 2021; Dong et al. 2022; Yan et al. 2022b). Tea is the most universally consumed healthy beverage worldwide. The potential health benefits of drinking have been extensively examined and are known to control blood sugar, prevent cardiovascular disease, boost the body's immunity and suppress tumour cell formation (Bag et al. 2022). The TPP, FAA, WLS, and CAF are the four leading indicators characterizing the tea quality, mainly related to the aroma, soup color, and taste of tea (Samynathan et al. 2021; Liao et al. 2022). Gao et al. (2012) indicated that bamboo charcoal and charcoal at 0.5% and 2.5% had no significant effect on TPP, but significantly reduced CAF. In this paper, TPP and CAF at 2.5% were significantly higher than CK at 16.64% and 51.69% (p < 0.05). This can be attributed to different biochar types and addition amounts (Nobaharan et al. 2022; Yan et al. 2022b). Similar to a previous report (Zhao et al. 2022), biochar application (5.0%) tended to increase the WLS while increasing the TPP. For WLS and CAF, the results of Zhang et al. (2022) and Li et al. (2021) supported that biochar could help to improve them. Studies have shown that the appropriate content of TPP was around 20.0%, and too high would lead to bitterness and astringency directly affecting the quality of tea (Liao et al. 2022). This study showed that the content of TPP in each treatment fluctuated between 14.24% and 18.31%, and 5.0% biochar addition had the best effect on improving TPP. However, Li et al. (2021) showed that the TPP under different biochar treatments was not significantly different, fluctuating between 13.50% and 14.58%. The FAA endows tea "umami" taste, its content in the tea range of 1.1–6.5%, and the higher the content of the better quality (Chen and Zhou 2005). In the present study, the FAA gradually decreased with biochar amounts, and was significantly higher in 0.5% treatment than in the other treatments (Fig. 2). This is similar to the study of Lin et al. (2022), which showed that a small amount of biochar addition is more effective for FAA. Notably, Luo et al. (2019) found that the addition of exogenous Ca may affect the content of TPP, FAA, CAF, gallic acid, and soluble sugars due to the synergistic effect with F, thus affecting the tea quality. In general, based on the purpose of tea quality improvement, 5.0–8.0% biochar application should be considered in tea garden practices.
Tea quality is closely related to soil nutrients (Zhao et al. 2022). Biochar is reported to help increase the input of soil K, Ca, and Mg, and promote the enrichment of nutrients in tea plants, which contributes to the improvement of tea quality (Zhao et al. 2015; Chen et al. 2021; Li et al. 2021). In this paper, biochar enhanced soil fertility metrics, including N, P, K, NH4+, AP, and AK (Additional file 1: Fig. S2), as well as promoting the uptake of available nutrients such as N, P and K by tea plants (Additional file 1: Fig. S3). It has been shown that FAA is a nitrogenous organic matter whose content depends on the enrichment of N in leaves (Yang et al. 2018; Tang et al. 2022). The results are not unrelated to the N nutrient contents of leaves in those treatments. Our results showed that TPP, WLS and CAF were positively correlated with soil exchangeable base cations (Fig. 3). With the increase of biochar, the tea Ws-F contents decreased and the tea quality improved. This is consistent with the results obtained by Yang et al. (2015) and Cai et al. (2016), who reported that excessive F accumulation could stress tea plants and affect tea quality. Chen et al. (2021) indicated that the TN content in tea garden soil increased with increasing biochar application, which suggests that biochar is beneficial in improving soil N retention. Wang et al. (2018) and Yan et al. (2021) also found that biochar can promote tea growth by alleviating soil acidification and reducing heavy metal availability. In addition, it should not be overlooked that the application of biochar also increases the abundance of beneficial bacteria and enzyme activity in the soil, thus improving the soil ecosystem function and providing a more friendly environment for the growth of tea plants (Jiang et al. 2021; Yang et al. 2021). In conclusion, these findings suggest that the soil environmental changes brought about by proper biochar amounts have a good prospect for improving tea quality.
4.3 Soil water-soluble F, pH, and exchangeable base cations affect tea F accumulation
F in tea plants mainly comes from the soil, and soil F bioavailability plays an essential role in the absorption and enrichment of F by tea leaves (Yi et al. 2017). Soil Ws-F is the main fraction absorbed and utilized by tea leaves (Xie et al. 2001; Sun et al. 2022). The results showed that 0.5–2.5% biochar reduced soil Ws-F content compared to CK, although the difference was insignificant (Table 1). However, biochar enhanced the F solubility at 5.0–10.0% application rates. This is because large amounts of biochar raised the soil pH and promoted the desorption of F from the soil solid phase (Evans and Jacobs 2020). This result contrasts with that of Gao et al. (2012), which reported that the addition of 5.0% biomass materials significantly reduced soil Ws-F content in a tea garden in Southern China. In this case, biochar modification can be used to improve the adsorption capacity. Fan et al. (2022) reported that 12.0% (w/w) trivalent metals-modified-biochar in F-contaminated soil could reduce Ws-F by up to 90.17%. The presence of F in soil is diverse and in dynamic change, and the determination of soil F fraction at different biochar amounts can reflect the change of soil F bioavailability, which is important for studying the migration of F from soil to tea plant (Gao et al. 2012; Yi et al. 2017; Long et al. 2021). The different fractions of F in soil are in dynamic balance, and the bioavailable Ws-F and Ex-F could be transformed into other fractions of F under the influence of external conditions (Cai et al. 2013). There was a highly remarkable negative correlation between soil Ws-F and soil Fe/Mn-F (p < 0.01), which could be found by correlation analysis (Fig. 3). Soil Ws-F is readily absorbed by plants, so converting it to a non-absorbable form could reduce the potential risk of F accumulation in plants (Li et al. 2018). Changing the dynamic equilibrium of insoluble, exchangeable and soluble F in the soil would affect F uptake by tea roots (Xu et al. 2006). In a previous study, different additions of biomass material significantly increased the soil Fe/Mn-F content and significantly decreased the soil Ws-F and Ex-F and tea Ws-F contents (p < 0.05) (Gao et al. 2012). Differences in F fraction distribution and content in soil following the application of biochar might be due to biochar and soil species in this study.
Furthermore, the study found that tea Ws-F was significantly negatively correlated with soil Ws-F (-0.59), contrary to previous findings (Xie et al. 2007; Long et al. 2021). However, other authors also found no significant correlation between soil and plant Ws-F in their experiments (Jha et al. 2008; Wang et al. 2012). A possible explanation for this is related to the different amounts of biochar causing different soil F solubility. It is reported that soil pH is the critical geochemical factor that determines the bioavailability of soil F and their transport into tea leaves (Yi et al. 2017). As our results show, the tested soil pH increased by 0.81–1.63 units, higher than in previous studies (Gao et al. 2012; Ghassemi-Golezani and Farhangi-Abriz 2019), which was mainly due to the high amount of biochar used in this study. Under high pH conditions, high levels of OH− in soil solution compete with F for sorption and may displace F from soil sorption sites, leading to an increase in Ws-F because they have almost the same diameter (Wang et al. 2012; Li et al. 2017). The OH− content increase led to a rise in soil pH, thus enhancing the solubility of F, which was also reflected by the correlation between soil pH and Ws-F (Fig. 3). Ruan et al. (2004) found that lime treatment increased soil pH and Ws-F content. Zhou et al. (2014) also concluded the same conclusion that soil pH and Ws-F have a positive correlation. However, it has also been noted that the correlation between soil Ws-F and pH is insignificant or even becomes negative (Cai et al. 2016; Gan et al. 2021). It is worth noting that biochar itself also contains F which may increase soil input risk. In our study, the Ws-F content of biochar and soil was 2.67 and 2.90 mg kg−1 (Additional file 1: Table S1), respectively, and the Ws-F content after mixing the highest biochar application (0.7 kg) with soil (7.0 kg) was lower than that of CK: (2.67 × 0.7 + 2.9 × 7) ÷ 7.7 = 2.88 < 2.90 mg kg−1. Hence, the risk of Ws-F; leaching from the biochar itself should be very low. Studies have shown that the tea Ws-F increased significantly with increasing pH when soil pH exceeded 5.0 (Sun et al. 2022). In this study, tea Ws-F was significantly negatively correlated with soil pH (−0.62) (Fig. 3). This indicates that although biochar increased soil Ws-F content, it decreased the transport of WS-F from soil to tea leaves, which may be related to some mechanism that will be discussed in the next 4.5.
The increase in soil pH is mainly related to introducing a lot of exchangeable base cations with the increase of biochar application (Fig. 3). Previous studies have shown a highly significant positive correlation between exchangeable K+, Na+, Ca2+, and Mg2+ and Ws-F in soil and tea (Yi et al. 2017). However, tea Ws-F contents showed a highly significant negative correlation with soil base ions in this study (p < 0.01). Ruan et al. (2004) found that adding Ca(NO3)2 or CaO to soil increased soil Ws-F content, but significantly decreased tea Ws-F content, which may be related to soil Ca2+ on the permeability of tea cell wall. Luo et al. (2019) also found that the effect of exogenous Ca on F tolerance in tea may be related to intracellular ion homeostasis (e.g., B, Al, Cu, and Zn). Ca2+ plays an important role in plant ion uptake as a ubiquitous second messenger (Luan 2009). Zhang et al. (2015) demonstrated that endogenous Ca2+ and CaM in tea plants contributed to F accumulation in tea rootpromoted by Al3+ pretreatment. Similar result showed that Ca2+-CaM was involved in the inhibition of F accumulation in tea plants by the anion channel inhibitor NPPB (Zhang et al. 2016). According to this paper, soil exchangeable Ca2+ content gradually increased with the increase of biochar amounts. Tea Ws-F was significantly negatively correlated with soil exchangeable Ca2+, with a coefficient of −0.78, higher than K+ and Mg2+. Therefore, we infer that soil exchangeable Ca2+ may be involved in biochar inhibition of Ws-F accumulation in tea leaves. Numerous studies have shown that increasing the content of soluble Ca2+ in soil by applying acidic soil amendments such as Nano-hydroxyapatite (NHAP), flue gas desulphurisation gypsum, and calcium oxide (CaO) could limit the migration of soil Ws-F to the above-ground parts of plants (Ruan et al. 2004; Alvarez-Ayuso et al. 2011; Gan et al. 2021). In any case, more intensive experiments are needed to investigate whether the increase in exchangeable Ca2+ brought about by biochar application is associated with reduced tea F accumulation.
4.4 Soil exchangeable aluminum affects tea F accumulation
In acidic soil solutions (pH < 6.0) in tea gardens, Al3+ could complex with F−, mainly in the form of AlF2+, AlF2+, and AlFx(3−x)− (Xie et al. 2007). In soil-tea ecosystems, the chemical behavior related to F and Al has been of interest because tea leaves absorb F mainly as F-Al complexes from the soil and transfer them to the leaves in this form (Xie et al. 2001; Yang et al. 2016). Soil-free Al3+ (e.g., Ex-Al) contributes to improving the absorption of soil F by tea plants and increases F enrichment of tea leaves (Zhao et al. 2015; Yang et al. 2018; Long et al. 2021). Li et al. (2016) and Fung et al. (1999) found that soil Ex-Al content was closely related to soil F transformation and migration. The acidic soils are known to enrich more Al, which affects the soil F components (An et al. 2015; Barrow et al. 2016). As far as we know, the dynamic effects of different biochar application rates on free Al3+ and F− in tea garden soils are unclear. In this study, soil Ex-Al showed an extremely significantly negative correlation with Ws-F and Ex-F (p < 0.01) and decreased significantly with the increase of biochar content (p < 0.05) (Fig. 3). Ex-Al is the most abundant and active Al form that could be extracted from tea garden soil, and is an important Al form that binds to Ws-F and is also the main source of aluminum in mature tea leaves (Xie et al. 2007). Biochar applied to acidic soils has been reported to reduce the bioavailability of soil Al and decrease plant toxicity (Dong et al. 2022). Dai et al. (2014) found that 3.0% swine manure and rape straw biochar reduced Ex-Al contents in acid soils by 99.0% and 90.0%, respectively. The Ws-F content of tea leaves was positively correlated with soil Ex-Al (r = 0.89, p < 0.05), further confirming that the available Al content in the soil might be able to predict the F contents in tea leaves (Xie et al. 2007). However, some reports (Ruan et al. 2003; Xie et al. 2007) mentioned that tea F and soil Ws-Al had the same change trend. In any case, the mechanism of F-Al complexes uptake and transport from soil to tea still lacks intuitive and strong evidence, especially under the conditions influenced by Ex-Al, and further studies are needed.
4.5 Fluoride-aluminum complexes affect tea F accumulation
The tea plant acts as an Al hyperaccumulator, and the presence of Al can affect the accumulation of tea F (Ruan et al. 2003; Yang et al. 2016). In recent years, several studies have revealed that the combined absorption of Al and F by tea plants, thereby eliminating the toxicity of F and Al alone, may be an important physiological mechanism for the high F enrichment of leaves (Ruan et al. 2003; Shu et al. 2003; Xie et al. 2007). Fu et al. (2022) and Gao et al. (2014) also revealed that internal tolerance mechanisms in tea plants can convert the absorbed Al and F into nontoxic or less toxic complexes as a way to reduce Al and F toxicity in the body. Long et al. (2021) mentioned that applying CO(NH2)2 to the tea garden soil could simultaneously enrich tea F and tea Al. However, information on the effect of biochar addition on Ws-Al in tea plants and, thus, on tea F accumulation remains limited. Therefore, it is imperative to study the impact of biochar on the content of Ws-Al in tea plants. The content distribution of Ws-Al in tea plant organs is consistent with previous reports in this study (Shu et al. 2003; Karak et al. 2015). Correlation analysis showed that the relationship between Ws-F and Ws-Al in tea roots, stems and leaves (p < 0.01) was significantly positive. The tea Ws-Al and Ws-F contents in each treatment followed the same accumulation trend and similar results were reported in previous studies (Xie et al. 2007; Long et al. 2021). It has been proposed that Al and F exist together in tea plant and that F-Al complexes are the main form of soil F uptake by tea plants compared to F- alone (Ding and Huang 1991; Nagata et al. 1993; Yang et al. 2016). Niu et al. (2020a) also reported that exogenous selenium (Se) treatment inhibited Ws-F accumulation in tea leaves, which may be related to the fact that the application of exogenous Se reduced the Al content in tea and thus the formation of F-Al complexes. Long et al. (2021) revealed that the changing trend of tea T-F at the same growth stage was consistent with the F and Al molar ratio, and the higher molar ratio of soil F and Al promoted the T-F accumulation of tea leaves. For now, there is a lack of quantitative determination of soil F-Al complexes, and the transfer and enrichment of soil F-Al complexes under biochar will only be inferred from the fluctuation of Ws-F and Ws-Al content in tea plants. Thus, the results above only explain that tea leaves probably absorb both F and Al combined in F-Al complexes (Ruan et al. 2003; Pan et al. 2020), and further research is needed on the specific mechanism of F absorption and enrichment by tea leaves.
Based on previous studies, it can be concluded that possible but necessary methods to reduce tea F bioavailability in tea gardens mainly include: (1) limiting soil Ws-F to reduce tea plant uptake through adsorbents such as charcoal, bamboo charcoal, and nano-hydroxyapatite (Gao et al. 2012; Gan et al. 2021); (2) chelating soil Al3+ by adsorbents to prevent the formation of F-Al complexes to reduce Ws-F uptake by tea plants, such as polyphenol-Ce adsorbents (Zhao et al. 2015). It was demonstrated that both of the above methods could significantly reduce the accumulation of Ws-F in tea leaves. Long et al. (2021) similarly concluded that the key to reducing tea F content in tea gardens is to mitigate soil acidification and reduce the content of F-Al complexes in tea garden soil. In this study, the correlation analysis showed that the Ws-Al contents of tea roots and leaves were significantly positively correlated with that in soil Ex-Al (p < 0.01) (Fig. 3). Xie et al. (2007) also found this phenomenon. Combining previous reports and current results, we can infer that although the extensive use of biochar increased soil Ws-F release, the soil Ex-Al is reduced accordingly, thereby reducing the formation of F-Al complexes and thus reducing the enrichment of Ws-F by tea leaves. This speculation may be supported to some extent by the fact that tea Ws-Al and Ws-F have the same increasing and decreasing trend at different biochar amounts (Fig. 1). However, effects on F-Al complexes in soil and tea plants with different biochar amounts remain to be further investigated because there is no practical method to analyze the F-Al complexes. In conclusion, whether the indirect reduction of F-Al complexes by biochar contributes to the inhibition of F accumulation in tea leaves still needs to be clarified in future studies. This information obtained from this study will provide useful references for developing strategies to reduce tea F accumulation in agricultural practices.
4.6 Environmental implications of this study
Every year, billions of tons of agricultural waste (e.g., straw) are produced worldwide. It makes more sense to convert them into biochar than to wasteful and polluting incineration (Hossain et al. 2020; Bhattacharyya et al. 2021). Biochar is considered to be a better amendment with little adverse effects on a wider environment as well as animal and human health (Liu et al. 2022). Many researchers have demonstrated the potential of biochar as a low-cost adsorbent, which has stimulated a growing research surge in soil remediation (Tang et al. 2020; Liu et al. 2021). Of course, the cost of preparation has to be considered in the process of using biochar. As biochar research has evolved, production methods are becoming more and more efficient. The lowest biochar production costs are reported to be close to zero (Maroušek 2014; Maroušek et al. 2019).
In recent years, biochar has shown benefits as a soil amendment in tea gardens and is considered an effective option for reducing nitrogen leaching and mitigating soil acidification due to its usability and sustainability (Chen et al. 2021; Li et al. 2021). However, there are few and unsystematic studies on biochar in reducing tea F and improving tea quality. In addition to the biochar and soil conditions themselves, the amount of biochar applied also affects its remediation effect. Controlling the amount is more convenient for production practice than controlling the feedstock and preparation conditions (Ali et al. 2020; Wang et al. 2022c). Therefore, our current study emphasizes the importance of different biochar amounts, especially at the high dose of 10.0%. Based on the comprehensive analysis of all indicators, our study clearly showed that 5.0–8.0% biochar treatment was the most suitable ratio for reducing tea F accumulation and improving tea quality. Compared to the high cost of adsorbents in previous studies (Zhao et al. 2015; Gan et al. 2021), the cost of biochar is acceptable, especially considering the high value of tea products. The information obtained in this study provides a more comprehensive understanding of the application of biochar in tea garden ecosystems. Admittedly, the current results are short-term effects, and we need to follow up and report long-term as well as field experimental results accordingly. In addition, more factors including biochar modification, tea biomass, cost–benefit analysis are required to consider in the next study.
5 Conclusions
In summary, the results of the pot experiment suggested that the application of biochar could decrease tea F enrichment of tea leaves and improve tea quality to varying degrees. The F content in tea stems was reduced among any amounts of biochar treatments, but there was no significant effect on the F accumulation in tea roots. Small amounts of biochar (0.5–2.5%) addition favored the fixation of soil Ws-F. However, high amounts of biochar (5.0–10.0%) instead increased the soil Ws-F solubility due to the increase of soil pH. Further analysis showed that the higher biochar application could increase soil exchangeable Ca2+ contents and decrease soil Ex-Al contents, which inhibit the uptake and accumulation of tea F to a certain extent. This may be an essential mechanism to better understand the biochar-induced reduction of F enrichment in tea leaves, which provides a new perspective to explain the effect of different amounts of biochar on the inhibition of different accumulations of F in tea leaves. The improvement of tea garden soil quality after biochar application is beneficial to the uptake of tea plant nutrients, which can improve tea quality (e.g., tea polyphenols and caffeine). In general, biochar is an acceptable tea garden soil amendment to reduce tea F content and improve tea quality, which plays a role in different amounts. Further research and long-term field trials are necessary to assess the effects of biochar on tea F accumulation and tea quality.
Novelty statement
This study investigated the effect of biochar application on F accumulation in tea leaves during the growth period, especially high amount of biochar application, and speculated the potential mechanism of reduction through pot experiments. Our results showed that biochar application could significantly reduce Ex-Al in soil, thus preventing the formation of F-Al complexes from F and eventually effectively inhibiting the accumulation of F in tea leaves. In addition, biochar was also beneficial to promote nutrient uptake, which in turn improved tea quality. However, it is recommended that the results obtained from the pot experiment be validated under field conditions.
Availability of data and materials
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
References
Ali A, Shaheen SM, Guo D, Li Y, Xiao R, Wahid F, Azeem M (2020) Apricot shell-and apple tree-derived biochar affect the fractionation and bioavailability of Zn and Cd as well as the microbial activity in smelter contaminated soil. Environ Pollut 264:114773. https://doi.org/10.1016/j.envpol.2020.114773
Alvarez-Ayuso E, Gimenez A, Ballesteros JC (2011) Fluoride accumulation by plants grown in acid soils amended with flue gas desulphurisation gypsum. J Hazard Mater 192:1659–1666. https://doi.org/10.1016/j.jhazmat.2011.06.084
An J, Lee HA, Lee J, Yoon HO (2015) Fluorine distribution in soil in the vicinity of an accidental spillage of hydrofluoric acid in Korea. Chemosphere 119:577–582. https://doi.org/10.1016/j.chemosphere.2014.07.043
Azeem M, Ali A, Jeyasundar PGSA, Li Y, Abdelrahman H, Latif A, Li R (2021) Bone-derived biochar improved soil quality and reduced Cd and Zn phytoavailability in a multi-metal contaminated mining soil. Environ Pollut 277:116800. https://doi.org/10.1016/j.envpol.2021.116800
Bag S, Mondal A, Majumder A, Banik A (2022) Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem 371:131098. https://doi.org/10.1016/j.foodchem.2021.131098
Barrow NJ, Debnath A, Chatterjee S (2016) Effect of pH and prior phosphate application on the reaction of fluoride with soils from northern India. Eur J Soil Sci 67:294–302. https://doi.org/10.1111/ejss.12337
Bhattacharyya P, Bisen J, Bhaduri D, Priyadarsini S, Munda S, Chakraborti M, Adak T (2021) Turn the wheel from waste to wealth: economic and environmental gain of sustainable rice straw management practices over field burning in reference to India. Sci Total Environ 775:145896. https://doi.org/10.1016/j.scitotenv.2021.145896
Cai HM, Peng CY, Chen J, Hou RY, Wan XC (2013) Chemical form changes of exogenous water solution fluoride and bioavailability in tea garden Soil. Environ Sci 34:4440–4446. https://doi.org/10.13227/j.hjkx.2013.11.049. (in Chinese)
Cai HM, Peng CY, Chen J, Hou RY, Gao HJ, Wan XC (2014) X-ray photoelectron spectroscopy surface analysis of fluoride stress in tea (Camellia sinensis (L.) O. Kuntze) leaves. J Fluorine Chem 158:11–15. https://doi.org/10.1016/j.jfluchem.2013.11.012
Cai HM, Dong YY, Li YY, Li DX, Peng CY, Zhang ZZ, Wan XC (2016) Physiological and cellular responses to fluoride stress in tea (Camellia sinensis) leaves. Acta Physiol Plant 38:144. https://doi.org/10.1007/s11738-016-2156-0
Cao J, Bai XX, Zhao Y, Liu J, Zhou D, Fang S, Jia M (1996) Fluorosis induced by drinking brick tea. Fluoride 29:139–143
Chen L, Zhou ZX (2005) Variations of main quality components of tea genetic resources [Camellia sinensis (L.) O. Kuntze] preserved in the China National Germplasm Tea Repository. Plant Food Hum Nutr 60:31–35. https://doi.org/10.1007/s11130-005-2540-1
Chen P, Liu Y, Mo C, Jiang Z, Yang J, Lin J (2021) Microbial mechanism of biochar addition on nitrogen leaching and retention in tea soils from different plantation ages. Sci Total Environ 757:143817. https://doi.org/10.1016/j.scitotenv.2020.143817
Chu Y, Liu Y, Guo N, Lou Q, Wang L, Huang W, Wu L (2021) Association between ALOX15 gene polymorphism and brick-tea type skeletal fluorosis in Tibetans, Kazaks and Han, China. Int J Environ Heal R 31:421–432. https://doi.org/10.1080/09603123.2019.1666972
Dai ZM, Li RB, Muhammad N, Brookes PC, Wang HZ, Liu XM, Xu JM (2014) Principle component and hierarchical cluster analysis of soil properties following biochar incorporation. Soil Sci Soc Am J 78:205–213. https://doi.org/10.2136/sssaj2013.05.0199
Das S, de Oliveira LM, da Silva E, Liu Y, Ma LQ (2017) Fluoride concentrations in traditional and herbal teas: health risk assessment. Environ Pollut 231:779–784. https://doi.org/10.1016/j.envpol.2017.08.083
Ding RX, Huang X (1991) Biogeochemical cycle of aluminium and fluorine in tea garden soil system and its relationship to soil acidification. Acta Pedolog Sin. 28:229–236 (in Chinese)
Dong Y, Yu YC, Wang RH, Chang E, Hong ZN, Hua H, Liu H (2022) Insights on mechanisms of aluminum phytotoxicity mitigation by canola straw biochars from different regions. Biochar. https://doi.org/10.1007/s42773-022-00179-6
Evans A, Jacobs MB (2020) Oxalate-fluoride anion exchange in alpine tundra soil: impact on aluminium transport. Eur J Soil Sci 72:1219–1230. https://doi.org/10.1111/ejss.13020
Fan C, Yin N, Cai X, Du X, Wang P, Liu X, Li Y (2022) Stabilization of fluorine-contaminated soil in aluminum smelting site with biochar loaded iron-lanthanide and aluminum-lanthanide bimetallic materials. J Hazard Mater 426:128072. https://doi.org/10.1016/j.jhazmat.2021.128072
Fu Z, Jiang X, Kong D, Chen Y, Zhuang J, Han M, Shi Y (2022) Flavonol–aluminum complex formation: enhancing aluminum accumulation in tea plants. J Agr Food Chem. https://doi.org/10.1021/acs.jafc.2c04963
Fung K, Zhang Z, Wong J, Wong MH (1999) Fluoride contents in tea and soil from tea plantations and the release of fluoride into tea liquor during infusion. Environ Pollut 104:197–205. https://doi.org/10.1016/S0269-7491(98)00187-0
Gan CD, Jia YB, Yang JY (2021) Remediation of fluoride contaminated soil with nano-hydroxyapatite amendment: Response of soil fluoride bioavailability and microbial communities. J Hazard Mater 405:124694. https://doi.org/10.1016/j.jhazmat.2020.124694
Gao H, Zhang Z, Wan X (2012) Influences of charcoal and bamboo charcoal amendment on soil-fluoride fractions and bioaccumulation of fluoride in tea plants. Environ Geochem Health 34:551–562. https://doi.org/10.1007/s10653-012-9459-x
Gao HJ, Zhao Q, Zhang XC, Wan XC, Mao JD (2014) Localization of fluoride and aluminum in subcellular fractions of tea leaves and roots. J Agr Food Chem 62:2313–2319. https://doi.org/10.1021/jf4038437
Ghassemi-Golezani K, Farhangi-Abriz S (2019) Biochar alleviates fluoride toxicity and oxidative stress in safflower (Carthamus tinctorius L.) seedlings. Chemosphere 223:406–415. https://doi.org/10.1016/j.chemosphere.2019.02.087
Guo W, Lin X, Jin LL, Hu SH (2020) Single quadrupole inductively coupled plasma-mass spectrometry for the measurement of fluorine in tea infusions and its health risk assessment. J Food Compos Anal 86:103378. https://doi.org/10.1016/j.jfca.2019.103378
Hossain MZ, Bahar MM, Sarkar B, Donne SW, Ok YS, Palansooriya KN, Kirkham MB (2020) Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2:379–420. https://doi.org/10.1007/s42773-020-00065-z
Huang C, Zhang H, Zeng W, Ma J, Zhao S, Jiang Y, Huang C (2020) Enhanced fluoride adsorption of aluminum humate and its resistance on fluoride accumulation in tea leaves. Environ Technol 41:329–338. https://doi.org/10.1080/09593330.2018.1498135
Jha SK, Nayak AK, Sharma YK, Mishra VK, Sharma DK (2008) Fluoride accumulation in soil and vegetation in the vicinity of brick fields. B Environ Contam Tox 80:369–373. https://doi.org/10.1007/s00128-008-9391-z
Jiang Y, Wang X, Zhao Y, Zhang C, Jin Z, Shan S, Ping L (2021) Effects of biochar application on enzyme activities in tea garden Soil. Front Bioeng Biotech 9:728530. https://doi.org/10.3389/fbioe.2021.728530
Jiang X, Li WW, Han M, Chen G, Wu J, Lai S, Fu Z (2022) Aluminum-tolerant, growth-promoting endophytic bacteria as contributors in promoting tea plant growth and alleviating aluminum stress. Tree Physiol 42:1043–1058. https://doi.org/10.1093/treephys/tpab159
Jun L, Wei H, Aili M, Juan N, Hongyan X, Jingsong H, Yunhua Z (2020) Effect of lychee biochar on the remediation of heavy metal-contaminated soil using sunflower: a field experiment. Environ Res 188:109886. https://doi.org/10.1016/j.envres.2020.109886
Kabir H, Gupta AK, Tripathy S (2020) Fluoride and human health: systematic appraisal of sources, exposures, metabolism, and toxicity. Crit Rev Env Sci Tec 50:1116–1193. https://doi.org/10.1080/10643389.2019.1647028
Karak T, Sonar I, Paul RK, Frankowski M, Boruah RK, Dutta AK, Das DK (2015) Aluminium dynamics from soil to tea plant (Camellia sinensis L.): is it enhanced by municipal solid waste compost application? Chemosphere 119:917–926. https://doi.org/10.1016/j.chemosphere.2014.08.067
Koblar A, Tavcar G, Ponikvar-Svet M (2012) Fluoride in teas of different types and forms and the exposure of humans to fluoride with tea and diet. Food Chem 130:286–290. https://doi.org/10.1016/j.foodchem.2011.07.037
Kumar R, Sharma P, Yang W, Sillanpaa M, Shang J, Bhattacharya P, Vithanage M (2022) State-of-the-art of research progress on adsorptive removal of fluoride-contaminated water using biochar-based materials: practical feasibility through reusability and column transport studies. Environ Res 214:114043. https://doi.org/10.1016/j.envres.2022.114043
Li SY, Li HX, Yang CL, Wang YD, Xue H, Niu YF (2016) Rates of soil acidification in tea plantations and possible causes. Agr Ecosyst Environ 233:60–66. https://doi.org/10.1016/j.agee.2016.08.036
Li Y, Jiang HT, Yang XZ (2017) Fluorine follows water: effect on electrical conductivity of silicate minerals by experimental constraints from phlogopite. Geochim Cosmochim Ac 217:16–27. https://doi.org/10.1016/j.gca.2017.08.020
Li Y, Wang S, Zhang Q, Zang F, Nan Z, Sun H, Huang W (2018) Accumulation, interaction and fractionation of fluoride and cadmium in sierozem and oilseed rape (Brassica napus L.) in northwest China. Plant Physiol Bioch 127:457–468. https://doi.org/10.1016/j.plaphy.2018.04.017
Li CJ, Yang WH, Zhou BQ, Zhang Y, Lin W, Xing SH (2021) Effects of biochar based fertilizer on soil nutrients, tea output and quality in an acidified tea field. Chin J Soil Sci 52:387–397. https://doi.org/10.19336/j.cnki.trtb.2020090301. (in Chinese)
Liao Y, Zhou X, Zeng L (2022) How does tea (Camellia sinensis) produce specialized metabolites which determine its unique quality and function: a review. Crit Rev Food Sci Nutr 62:3751–3767. https://doi.org/10.1080/10408398.2020.1868970
Lin S, Chen Z, Chen T, Deng W, Wan X, Zhang Z (2022) Theanine metabolism and transport in tea plants (Camellia sinensis L.): advances and perspectives. Crit Rev Biotechnol:1–15. https://doi.org/10.1080/07388551.2022.2036692
Liu YL, Luo HY, Tie BQ, Li DY, Liu ST, Lei M, Du HH (2021) The long-term effectiveness of ferromanganese biochar in soil Cd stabilization and reduction of Cd bioaccumulation in rice. Biochar 3:499–509. https://doi.org/10.1007/s42773-021-00113-2
Liu M, Almatrafi E, Zhang Y, Xu P, Song B, Zhou C, Zeng G (2022) A critical review of biochar-based materials for the remediation of heavy metal contaminated environment: applications and practical evaluations. Sci Total Environ 806:150531. https://doi.org/10.1016/j.scitotenv.2021.150531
Long H, Jiang Y, Li C, Liao S, Shi S, Huang C, Zhao S (2021) Effect of urea feeding on transforming and migrating soil fluorine in a tea garden of hilly region. Environ Geochem Health 43:5087–5098. https://doi.org/10.1007/s10653-021-00949-4
Luan S (2009) The CBL-CIPK network in plant calcium signaling. Trends Plant Sci 14:37–42. https://doi.org/10.1016/j.tplants.2008.10.005
Luo JL, Ni DJ, He C, Zhang SM, Liu SY, Du YR, Chen YQ (2019) Influence of exogenous calcium on the physiological, biochemical, phytochemical and ionic homeostasis of tea plants (Camellia sinensis (L.) O. Kuntze) subjected to fluorine stress. Plant Growth Regul 87:455–465. https://doi.org/10.1007/s10725-019-00478-2
Luo JL, Hu K, Qu FF, Ni DJ, Zhang HJ, Liu SY, Chen YQ (2021) Metabolomics analysis reveals major differential metabolites and metabolic alterations in tea plant leaves (Camellia sinensis L.) under different fluorine conditions. J Plant Growth Regul 40:798–810. https://doi.org/10.1007/s00344-020-10141-0
Lv HP, Lin Z, Tan JF, Guo L (2013) Contents of fluoride, lead, copper, chromium, arsenic and cadmium in Chinese Pu-erh tea. Food Res Int 53:938–944. https://doi.org/10.1016/j.foodres.2012.06.014
Lv D, Wang Z, Sun Y, Jin W, Wang Y, Zhou L, Zheng X (2021) The effects of low-dose biochar amendments on arsenic accumulation in rice (Oryza sativa L.). Environ Sci Pollut R 28:13495–13503. https://doi.org/10.1007/s11356-020-11572-8
Maroušek J (2014) Significant breakthrough in biochar cost reduction. Clean Technol Envir 16:1821–1825. https://doi.org/10.1007/s10098-014-0730-y
Maroušek J, Strunecký O, Stehel V (2019) Biochar farming: defining economically perspective applications. Clean Technol Envir 21:1389–1395. https://doi.org/10.1007/s10098-019-01728-7
Moon DH, Jo R, Koutsospyros A, Cheong KH, Park JH (2015) Soil washing of fluorine contaminated soil using various washing solutions. Bull Environ Contam Toxicol 94:334–339. https://doi.org/10.1007/s00128-014-1449-5
Nagata T, Hayatsu M, Kosuge N (1993) Aluminium kinetics in the tea plant using 27Al and 19F NMR. Phytochemistry 32:771–775. https://doi.org/10.1016/0031-9422(93)85202-3
Natarajan R, Al Qasmi F (2018) Biosorption of fluoride using Anogeissus dhofarica – effect of process variables and kinetic studies. Water Supply 18:1803–1809. https://doi.org/10.2166/ws.2017.247
Niu H, Zhan K, Xu W, Peng C, Hou C, Li Y, Hou R (2020a) Selenium treatment modulates fluoride distribution and mitigates fluoride stress in tea plant (Camellia sinensis (L.) O. Kuntze). Environ Pollut 267:115603. https://doi.org/10.1016/j.envpol.2020.115603
Niu HL, Peng CY, Zhu XD, Dong YY, Li YY, Tang LL, Wan XC (2020) Positron-emitting tracer imaging of fluoride transport and distribution in tea plant. J Sci Food Agric 100:3554–3559. https://doi.org/10.1002/jsfa.10367
Nobaharan K, Abtahi A, AsgariLajayer B, van Hullebusch ED (2022) Effects of biochar dose on cadmium accumulation in spinach and its fractionation in a calcareous soil. Arab J Geosci 15:336. https://doi.org/10.1007/s12517-022-09608-z
Pan J, Li D, Zhu J, Shu Z, Ye X, Xing A, Wen B (2020) Aluminum relieves fluoride stress through stimulation of organic acid production in Camellia sinensis. Physiol Mpl Biol Pla 26:1127–1137. https://doi.org/10.1007/s12298-020-00813-2
Peng CY, Xu XF, Ren YF, Niu HL, Yang YQ, Hou RY, Wan XC (2021) Fluoride absorption, transportation and tolerance mechanism in Camellia sinensis, and its bioavailability and health risk assessment: a systematic review. J Sci Food Agric 101:379–387. https://doi.org/10.1002/jsfa.10640
Peng CY, Chen J, Cai HM, Hou RY, Wan XC (2013) The kinetic characteristics of solution fluoride uptake by tea plant. Chin J Trop Crops 34:495–500. https://doi.org/10.3969/j.issn.1000-2561.2013.018. (in Chinese)
Rizzu M, Tanda A, Cappai C, Roggero PP, Seddaiu G (2021) Impacts of soil and water fluoride contamination on the safety and productivity of food and feed crops: a systematic review. Sci Total Environ 787:147650. https://doi.org/10.1016/j.scitotenv.2021.147650
Ruan JY, Wong MH (2001) Accumulation of fluoride and auminium related to different varieties of tea plant. Environ Geochem Health 23:53–63. https://doi.org/10.1023/A:1011082608631
Ruan JY, Ma LF, Shi YZ, Han WY (2003) Uptake of fluoride by tea plant (Camellia sinensis L) and the impact of aluminium. J Sci Food Agric 83:1342–1348. https://doi.org/10.1002/jsfa.1546
Ruan J, Ma L, Shi Y, Han W (2004) The impact of pH and calcium on the uptake of fluoride by tea plants (Camellia sinensis L.). Ann Bot-London 93:97–105. https://doi.org/10.1093/aob/mch010
Ruan J, Ma L, Shi Y (2006) Aluminium in tea plantations: mobility in soils and plants, and the influence of nitrogen fertilization. Environ Geochem Health 28:519–528. https://doi.org/10.1007/s10653-006-9047-z
Samynathan R, Thiruvengadam M, Nile SH, Shariati MA, Rebezov M, Mishra RK, Venkidasamy B (2021) Recent insights on tea metabolites, their biosynthesis and chemo-preventing effects: a review. Crit Rev Food Sci Nutr.1–20. https://doi.org/10.1080/10408398.2021.1984871
Satou R, Oka S, Sugihara N (2021) Risk assessment of fluoride daily intake from preference beverage. J Dent Sci 16:220–228. https://doi.org/10.1016/j.jds.2020.05.023
Sha JQ, Zheng DX (1994) Study on the fluorine content in fresh leaves of tea plant planted in Fujian Province. J Tea Sci 1:37–42. https://doi.org/10.13305/j.cnki.jts.1994.01.006. (in Chinese)
Shi RY, Ni N, Nkoh JN, Dong Y, Zhao WR, Pan XY, Li JY (2020) Biochar retards Al toxicity to maize (Zea mays L.) during soil acidification: the effects and mechanisms. Sci Total Environ 719:137448. https://doi.org/10.1016/j.scitotenv.2020.137448
Shu WS, Zhang ZQ, Lan CY, Wong MH (2003) Fluoride and aluminium concentrations of tea plants and tea products from Sichuan Province, PR China. Chemosphere 52:1475–1482. https://doi.org/10.1016/s0045-6535(03)00485-5
Sun H, Wen B, Wu Z, Xing A, Xu X, Chang Y, Guo G (2022) The performance of water-soluble fluoride transformation in soil–tea–tea infusion chain system and the potential health risk assessment. J Sci Food Agric 102:2893–2902. https://doi.org/10.1002/jsfa.11630
Sun YH, Wang WJ, Zhao ZY, Cai K, Zhao M (2017) Effects of biochar on adsorption characteristics of water-soluble fluorine in tea garden Soil. J Agric Resour Environ 34:376–383. https://doi.org/10.13254/j.jare.2017.0032. (in Chinese)
Tang J, Zhang L, Zhang J, Ren L, Zhou Y, Zheng Y, Luo L (2020) Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci Total Environ 701:134751. https://doi.org/10.1016/j.scitotenv.2019.134751
Tang S, Pan WK, Tang R, Ma QX, Zhou JJ, Zheng N, Wang J (2022) Effects of balanced and unbalanced fertilisation on tea quality, yield, and soil bacterial community. Appl Soil Ecol 175:104442. https://doi.org/10.1016/j.apsoil.2022.104442
Wang C, Yang ZF, Chen LX, Yuan XY, Liao QL, Ji JF (2012) The transfer of fluorine in the soil–wheat system and the principal source of fluorine in wheat under actual field conditions. Field Crop Res 137:163–169. https://doi.org/10.1016/j.fcr.2012.08.001
Wang Y, Yin RZ, Liu RH (2014) Characterization of biochar from fast pyrolysis and its effect on chemical properties of the tea garden soil. J Anal Appl Pyrol 110:375–381. https://doi.org/10.1016/j.jaap.2014.10.006
Wang J, Zhang BB, Tian Y, Zhang HC, Cheng Y, Zhang JB (2018) A soil management strategy for ameliorating soil acidification and reducing nitrification in tea plantations. Eur J Soil Biol 88:36–40. https://doi.org/10.1016/j.ejsobi.2018.06.001
Wang M, Li X, He WY, Li JX, Zhu YY, Liao YL, Yang JY (2019) Distribution, health risk assessment, and anthropogenic sources of fluoride in farmland soils in phosphate industrial area, southwest China. Environ Pollut 249:423–433. https://doi.org/10.1016/j.envpol.2019.03.044
Wang M, Zhang L, Liu Y, Chen D, Liu L, Li C, Kang KJ (2021) Spatial variation and fractionation of fluoride in tobacco-planted soils and leaf fluoride concentration in tobacco in Bijie City, Southwest China. Environ Sci Pollut R 28:26112–26123. https://doi.org/10.1007/s11356-020-11973-9
Wang DL, Fu YH, Yang JY (2022a) Enhanced fluoride stabilization by modified layered double hydroxides for the remediation of soil pollution: performance and mechanism. Water Air Soil Poll 233:471. https://doi.org/10.1007/s11270-022-05938-6
Wang XF, Li Y, Wang HR, Wang YZ, Biswas A, Wai Chau H, Liang JP (2022b) Targeted biochar application alters physical, chemical, hydrological and thermal properties of salt-affected soils under cotton-sugarbeet intercropping. CATENA 216:106414. https://doi.org/10.1016/j.catena.2022.106414
Wang Y, Shen X, Bian R, Liu X, Zheng J, Cheng K, Xuhui Z (2022c) Effect of pyrolysis temperature of biochar on Cd, Pb and As bioavailability and bacterial community composition in contaminated paddy soil. Ecotoxicol Environ Saf 247:114237. https://doi.org/10.1016/j.ecoenv.2022.114237
Xie ZM, Ye ZH, Wong MH (2001) Distribution characteristics of fluoride and aluminum in soil profiles of an abandoned tea plantation and their uptake by six woody species. Environ Int 26:341–346. https://doi.org/10.1016/S0160-4120(01)00010-1
Xie ZL, Chen Z, Sun WT, Guo XJ, Yin B, Wang JH (2007) Distribution of aluminum and fluoride in tea plant and soil of tea garden in Central and Southwest China. Chinese Geogr Sci 17:376–382. https://doi.org/10.1007/s11769-007-0376-3
Xu R, Wang Y, Zhao A, Ji G, Zhang H (2006) Effect of low molecular weight organic acids on adsorption and desorption of fluoride on variable charge soils. Environ Geochem Health 28:141–146. https://doi.org/10.1007/s10653-005-9023-z
Yan P, Shen C, Fan LC, Li X, Zhang LP, Zhang L, Han WY (2018) Tea planting affects soil acidification and nitrogen and phosphorus distribution in soil. Agr Ecosyst Environ 254:20–25. https://doi.org/10.1016/j.agee.2017.11.015
Yan HL, Yang HX, Li KC, Zhu PF, Li XL, Li QL (2022a) Biochar addition modified carbon flux and related microbiota in cow manure composting. Waste Biomass Valori. https://doi.org/10.1007/s12649-022-01896-6
Yan SH, Zhang SL, Yan PK, Aurangzeib M (2022b) Effect of biochar application method and amount on the soil quality and maize yield in Mollisols of Northeast China. Biochar 4:56. https://doi.org/10.1007/s42773-022-00180-z
Yan P, Wu L, Wang D, Fu J, Shen C, Li X, Zhang L (2020) Soil acidification in Chinese tea plantations. Sci Total Environ 715:136963. https://doi.org/10.1016/j.scitotenv.2020.136963
Yan P, Shen C, Zou ZH, Fu JY, Li X, Zhang LP, Zhang L (2021) Biochar stimulates tea growth by improving nutrients in acidic soil. SCI Hortic-Amsterdam 283:110078. https://doi.org/10.1016/j.scienta.2021.110078
Yang X, Yu Z, Zhang BB, Huang J, Zhang YH, Fang FX, Li CL (2015) Effect of fluoride on the biosynthesis of catechins in tea [Camellia sinensis (L.) O. Kuntze] leaves. SCI Hortic-Amsterdam 184:78–84. https://doi.org/10.1016/j.scienta.2014.12.031
Yang Y, Liu Y, Huang CF, de Silva J, Zhao FJ (2016) Aluminium alleviates fluoride toxicity in tea (Camellia sinensis). Plant Soil 402:179–190. https://doi.org/10.1007/s11104-015-2787-8
Yang XD, Ni K, Shi YZ, Yi XY, Zhang QF, Fang L, Ma LF (2018) Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agr Ecosyst Environ 252:74–82. https://doi.org/10.1016/j.agee.2017.10.004
Yang WH, Li CJ, Wang SS, Zhou BQ, Mao YL, Rensing C, Xing SH (2021) Influence of biochar and biochar-based fertilizer on yield, quality of tea and microbial community in an acid tea orchard soil. Appl Soil Ecol 166:104005. https://doi.org/10.1016/j.apsoil.2021.104005
Yang J, Zheng ZC, Li TX, Ye DH, Wang YD, Huang HG, Yu HY (2022) Variations in aluminum fractions within soils associated with different tea (Camellia sinensis L.) varieties: insights at the aggregate scale. Plant Soil. https://doi.org/10.1007/s11104-022-05563-6
Yao B, Chen X, Zhou K, Luo ZR, Li PP, Yang ZH, Zhou YY (2022) p-Arsanilic acid decontamination over a wide pH range using biochar-supported manganese ferrite material as an effective persulfate catalyst: performances and mechanisms. Biochar 4(1):1–13. https://doi.org/10.1007/s42773-022-00158-x
Yi X, Qiao S, Ma L, Wang J, Ruan J (2017) Soil fluoride fractions and their bioavailability to tea plants (Camellia sinensis L.). Environ Geochem Health 39:1005–1016. https://doi.org/10.1007/s10653-016-9868-3
Yi X, Ji L, Hu Z, Yang X, Li H, Jiang Y, He T (2022) Organic amendments improved soil quality and reduced ecological risks of heavy metals in a long-term tea plantation field trial on an Alfisol. Sci Total Environ 838:156017. https://doi.org/10.1016/j.scitotenv.2022.156017
Yin JL, Zheng ZC, Li TX, Zhang XZ, He SQ, Wang YD, Yu HY (2016) Effect of tea plantation age on the distribution of fluoride and its fractions within soil aggregates in the hilly region of Western Sichuan, China. J Soil Sediment 16:2128–2137. https://doi.org/10.1007/s11368-016-1409-2
Zhang L, Li Q, Ma LF, Ruan JY (2012) Characterization of fluoride uptake by roots of tea plants (Camellia sinensis (L.) O. Kuntze). Plant Soil 366:659–669. https://doi.org/10.1007/s11104-012-1466-2
Zhang XC, Gao HJ, Wu HH, Yang TY, Zhang ZZ, Mao JD, Wan XC (2015) Ca2+ and CaM are involved in Al3+ pretreatment-promoted fluoride accumulation in tea plants (Camellia sinesis L.). Plant Physiol Bioch 96:288–295. https://doi.org/10.1016/j.plaphy.2015.08.007
Zhang XC, Gao HJ, Yang TY, Wu HH, Wang YM, Zhang ZZ, Wan XC (2016) Anion channel inhibitor NPPB-inhibited fluoride accumulation in tea plant (Camellia sinensis) is related to the regulation of Ca2+, CaM and depolarization of plasma membrane potential. Int J Mol Sci 17:57. https://doi.org/10.3390/ijms17010057
Zhang R, Cheng L, Zhang T, Xu T, Li M, Yin W, Jiang Q (2019) Brick tea consumption is a risk factor for dental caries and dental fluorosis among 12-year-old Tibetan children in Ganzi. Environ Geochem Health 41:1405–1417. https://doi.org/10.1007/s10653-018-0216-7
Zhang MY, Zhang L, Riaz M, Xia H, Jiang CC (2021) Biochar amendment improved fruit quality and soil properties and microbial communities at different depths in citrus production. J Clean Prod 292:126062. https://doi.org/10.1016/j.jclepro.2021.126062
Zhang SH, Deng XJ, Zhao CS, Ding FH, Qiu YH, Jin AW (2022) Effects of bamboo char-based biochar application on tea quality and microbial community structure in tea plantation. J Nucl Agric Sci 36:1485–1495. https://doi.org/10.11869/j.issn.100-8551.2022.07.1485. (in Chinese)
Zhao SL, Liu Y, Ma J, Mao H, Lan T, Jiang YF, Liao Y (2015) Influence of fertilizers on fluoride accumulation in tea leaves and its remediation using polyphenol–Ce adsorbents. Rsc Adv 5:6085–6091. https://doi.org/10.1039/c4ra10361k
Zhao H, Fang HD, Cao YL, Ye X, Yang ZJ (2022) Improvement effect of composite conditioner on soil and tea quality in tea garden. J Jimei Univ Nat Sci 27:317–325. https://doi.org/10.19715/j.jmuzr.2022.04.04. (in Chinese)
Zhou M, Zhu SF, Liu YN, Wang H (2014) Electrokinetic remediation of fluorine-contaminated soil using approaching cathodes. Clean-Soil Air Water 42:1771–1775. https://doi.org/10.1002/clen.201200599
Zhu SF, Han DL, Zhou M, Liu YN (2016) Ammonia enhanced electrokinetics coupled with bamboo charcoal adsorption for remediation of fluorine-contaminated kaolin clay. Electrochim Acta 198:241–248. https://doi.org/10.1016/j.electacta.2016.03.033
Zhu J, Xing A, Wu Z, Tao J, Ma Y, Wen B, Zhu X (2019) CsFEX, a fluoride export protein gene from Camellia sinensis, alleviates fluoride toxicity in transgenic Escherichia coli and Arabidopsis thaliana. J Agr Food Chem 67:5997–6006. https://doi.org/10.1021/acs.jafc.9b00509
Acknowledgements
Thanks to Zedong Mo, Yingying Ye, Biao Yang, Shijie Yuan, Jiali Li, Jixiang Luo who helped us in setting up potted experiments, investigations, and laboratory analyses; Thanks to Mr. Yankai Kang for providing tea seedlings from Tea Research Institute of Hunan Province, Changsha, Hunan; Thanks to Miss. Cui Shang for assisting part of the experiments from Testing Center of Resource and Environment, Hunan Agricultural University; Thanks to Eceshi (www. Eceshi.com) for supporting the determination of biochar elements. Last but not least, we appreciate the editor and the anonymous reviewers for the valuable suggestions and insightful comments.
Funding
This work was supported by Chenzhou National Sustainable Development Agenda Innovation Demonstration Zone Construction Project (2021SFQ05); the General Project of Hunan Natural Science Foundation (2022JJ30291); the Special Project for the Construction of Modern Agricultural Industrial Technology Systems in Hunan Province (Xiang Nongfa) (2019105); Hunan Agricultural University "1515" Talent Project; and Postgraduate Scientific Research Innovation Project of Hunan Province (QL20210172).
Author information
Authors and Affiliations
Contributions
HW: Methodology, Investigation, Formal analysis, Writing-original draft. TH: Conceptualization, Data curation, Formal analysis, Visualization. MW: Conceptualization, Data curation, Formal analysis. YL: Writing-review and editing, Supervision, Founding acquisition. CS: Methodology, Supervision, Funding acquisition. HX: Writing-review and editing, Supervision, Project administration. YZ: Validation, Visualization, Supervision, Resources. ZL: Conceptualization, Supervision, Resources. All of the authors participated in the study and agreed to the content of the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Jun Meng
Supplementary Information
Additional file 1
: Table S1 Basic physicochemical properties of the selected soil and biochar. Table S2 Examples of studies using 10.0% biochar to treat different pollutants in soil. Fig. S1 Effects of biochar treatments on total fluoride in tea leaves. Fig. S2 Effects of biochar treatments on soil total nitrogen (N), total phosphorus (P), total potassium (K), ammonia nitrogen (NH4+), available phosphorus (AP) and available potassium (AK). Fig. S3 Effects of biochar treatments on tea plant tissues total nitrogen (TN), total phosphorus (TP) and total potassium (TK).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Hu, T., Wang, M. et al. Biochar addition to tea garden soils: effects on tea fluoride uptake and accumulation. Biochar 5, 37 (2023). https://doi.org/10.1007/s42773-023-00220-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00220-2