Abstract
Arsenic (As) is recognized as a persistent and toxic contaminant in the environment that is harmful to humans. Biochar, a porous carbonaceous material with tunable functionality, has been used widely as an adsorbent for remediating As-contaminated water and soils. Several types of pristine and modified biochar are available, and significant efforts have been made toward modifying the surface of biochars to increase their adsorption capacity for As. Adsorption capacity is influenced by multiple factors, including biomass pyrolysis temperature, pH, the presence of dissolved organic carbon, surface charge, and the presence of phosphate, silicate, sulfate, and microbial activity. Improved As adsorption in modified biochars is attributed to several mechanisms including surface complexation/precipitation, ion exchange, oxidation, reduction, electrostatic interactions, and surface functional groups that have a relatively higher affinity for As. Modified biochars show promise for As adsorption; however, further research is required to improve the performance of these materials. For example, modified biochars must be eco-friendly, cost-effective, reliable, efficient, and sustainable to ensure their widespread application for immobilizing As in contaminated water and soils. Conducting relevant research to address these issues relies on a thorough understanding of biochar modifications to date. This study presents an in-depth review of pristine and modified biochars, including their production, physicochemical properties, and As adsorption mechanisms. Furthermore, a comprehensive evaluation of biochar applications is provided in As-contaminated environments as a guide for selecting suitable biochars for As removal in the field.
Graphical Abstract

Highlights
-
The production, physicochemical properties, and As adsorption mechanisms of pristine and modified biochars were reviewed.
-
Pyrolysis temperature, pH, presence of dissolved organic carbon, surface charge, and microbial activity affect the As adsorption.
-
Modified biochars show promise for As adsorption due to combined mechanisms of complexation, ion exchange, oxidation, and reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Arsenic (As) is a toxic metalloid that occurs naturally worldwide. The inorganic forms of As are considered highly toxic, giving rise to significant global environmental concerns (Yang et al. 2017; Zhang et al. 2022a). The most dangerous inorganic As species, arsenate [As(V)] and arsenite [As(III)], have been shown to be present in both drinking water and soils (Oremland and Stolz 2003). Arsenic has become a critical threat to human health and the environment owing to its toxicity, non-biodegradability, persistence, bioaccumulation, and potential carcinogenic properties (Rahaman et al. 2021; Yang et al. 2019; Zhao and Luo 2018). Naturally occurring geochemical processes including weathering of rock, minerals, and volcanic emissions, and biological processes produce mobile As species from minerals that are released into the environment (Natasha et al. 2021; Rahaman et al. 2008). Human activities including smelting, mining, discharge of industrial wastewater and waste, pesticide application, and irrigation generate As contamination in the environment (Liu et al. 2019a, 2021; Sheng et al. 2012). Moreover, rapid urbanization and industrialization have contributed to significant and widespread environmental contamination by As.
The presence of elevated As concentrations in drinking water and groundwater is a crucial global health and environmental problem (Aftabtalab et al. 2022; Hussain et al. 2021; Shaji et al. 2021). The standard provisional guideline of the World Health Organization and the United States Environmental Protection Agency (USEPA) indicates an As threshold of 10 μg/L in drinking water (Ali et al. 2020). In numerous countries worldwide, As concentrations generally range between 140 and 1630 μg/L in contaminated wastewater and water (Shahid et al. 2020). However, As concentrations reach 2000 mg/L in the groundwater of the Ganges River alluvial deposits and in the Bengal Delta Plain (Brickson 2003). Arsenic contamination in public drinking water has already negatively affected several South and Southeast Asian countries, including Nepal, Bangladesh, Pakistan, India, and China (Li et al. 2021a, b; Niazi et al. 2018b; Shakoor et al. 2018), as well as the USA and Latin America (Bundschuh et al. 2021; Foster et al. 2019). Arsenic contamination in soils is also a global problem (Toth et al. 2016) that has severe influences on the environment and human health, particularly in India, Bangladesh, China, and Korea (Kwon et al. 2017; Shrivastava et al. 2017). Although the USEPA sets the maximum allowable As concentration at 24 mg kg-1 in soils (Singh et al. 2015), a wide range of contents (up to 250,000 mg kg-1) has been found in Bangladesh, West Bengal, and India (Mahimairaja et al. 2005; Mikutta et al. 2014; Shrivastava et al. 2015). The concentrations found in Lower Silesia in Southwestern Poland are up to 18,100 mg kg-1 (Singh et al. 2015). Considering the pollution status and risks associated with long-term As exposure, sustainable remediation strategies for environmental As contamination urgently need to be developed.
Removing or immobilizing As in water and soils remains problematic. Conventional removal techniques include ion exchange, precipitation, filtration, reverse osmosis, capacitive deionization, and membrane separation (Cuong et al. 2022; Fan et al. 2016; Niazi et al. 2018a, 2018b). Conventional materials include carbonaceous materials such as activated carbons and carbon nanotubes (CNTs), and other nanomaterials such as zerovalent iron (nZVI), and titanium dioxide nanoparticles (TiO2 NPs), which have been used for As remediation (Cai et al. 2019). These remediation methods and materials have disadvantages, such as high energy consumption rates, high costs, the generation of substantial amounts of waste, and incomplete contaminant removal (Mubarak et al. 2018). Unlike traditional As treatment technologies and materials, biochar has emerged as a promising and effective method and material that is cost-effective, highly efficient, simple to apply, and generates less waste (Mohanty 2017; Roy et al. 2017; Wei et al. 2019a). Increasing attention is being given to using biochar for its potential environmental benefits and uses when developing innovative As removal strategies, and biochar surface chemistry and As adsorption processes are also being extensively studied (Fig. 1).
Scientometrics visualizes the top 50 keywords for all peer-reviewed publications since 2009. The selected database was the Web of Science Core Collection with 538 papers retrieved based on the keywords (topics) “arsenic” and “biochar”. The data were analyzed using the keyword co-emergence feature built into VOSviewer, plotted in “Network Visualization,” “Overlay Visualization (year),” and “Density Visualization.” Each circle represents a keyword, and the size of the circle represents the number of times a pair of keywords appears together in a publication. The color-coded legend shows the average year each keyword appeared
Biochar indicates a super ability to immobilize As from the water and soils (Beiyuan et al. 2017; Yoo et al. 2018). Several types of biochar from waste biomass have been used to adsorb As(V) and As(III), including Tectona and Lagerstroemia speciose (Verma and Singh 2019), oak wood (Niazi et al. 2018b), perilla leaf (Niazi et al. 2018a), corn straw (He et al. 2018), chestnut shell (Zhou et al. 2017), perennial grass (Saikia et al. 2017), hybrid poplar wood, pine bark (Suliman et al. 2017), pinewood (Essandoh et al. 2015), rice husk (Agrafioti et al. 2014a), sewage sludge (Wongrod et al. 2018), Douglas fir (Navarathna et al. 2019), cotton stalks (Hussain et al. 2020), grape seeds (Fristak et al. 2018), waste plant litter (Verma and Singh 2019), peanut shells (Sattar et al. 2019), and Cassia fistula (Alam et al. 2018a). Indeed, the extensive engineering applications of biochar have been successfully applied in the in situ remediations of groundwater and soils. Notably, biochar has been used to immobilize of As in soils, can improve soil health, and is an environmentally benign ways of fixing carbon in soil (Jeffery et al. 2011). Such wide applications have led to a widespread use of biochar into natural environments, such that 1–40% of the organic carbon detected in soils being derived from biochar (Zhong et al. 2020). Therefore, biochar is a promising carbon material for As remediation and has received considerable interest as a treatment medium.
Biochar is easily produced from a variety of raw materials at low cost, and it is considered environmentally friendly (Bolan et al. 2022; Igalavithana et al. 2019; Shaheen et al. 2022a; Vithanage et al. 2017). Notably biochar has favorable physical/chemical surface properties (a porous structure and a high specific surface area) associated with high densities of reactive surface functional groups (Ahmad et al. 2014; Alam et al. 2018b; El-Naggar et al. 2019a, 2019b; Zimmerman et al. 2011). However, the adsorptive capacity of raw biochar for As is relatively low and, consequently, requires modification to improve adsorption performance (Benis et al. 2020). Modified biochar is considered a novel method to induce surface functionalities that promote As adsorption or immobilization (Rajapaksha et al. 2016; Yang et al. 2022a, 2022b). However, economically feasible biochar modification approaches are required to enhance adsorption capabilities. Chemically modified biochars with improved As contaminant adsorption capacity have already been developed (Alkurdi et al. 2019; Mohan and Pittman 2007; Shaheen et al. 2022c). In view of the increasing interest in biochar and its surface modifications, a more comprehensive understanding of the physicochemical properties and mechanisms that control As adsorption is needed.
Several review papers have been published on As remediation using biochar. For instance, pristine and modified/activated biochar have been compared for As removal from water (Srivastav et al. 2021), groundwater (Siddiq et al. 2022), both soils and water (Vithanage et al. 2017), and in the uptake by crops (Kumarathilaka et al. 2020). The key factors influencing biochar application for As removal and the significance of redox transformation reactions of As have also been discussed (Amen et al. 2020). Various biochar modification methods for aquatic As removal have been introduced, focusing on physical and chemical treatments (Benis et al. 2020). Despite such information being available, no study has presented a direct comparison between the application of a range of pristine and modified biochars for water and soil remediation, nor have their influencing factors and removal mechanisms been simultaneously described.
To address this gap, we conducted a comparative study of the effects of pristine and modified biochar. The aim of this review was to determine the gaps in the existing research and the problems that must be solved by future studies. All available modification trials were reviewed for biochar and various modified biochars were compared for their efficiency for As removal in water and immobilization in soils, relative to pristine biochars. In view of the serious effects of As contamination, the development of more innovative remediation strategies was considered, as this is crucial to paving the way for the establishment of sustainable As mitigation measures at a global scale.
2 Biochar for the removal of arsenic from contaminated water
2.1 Pristine biochars
Biochar is used in water as an environmentally friendly adsorbent. Different feedstock types, including solids, industrial by-products, and agricultural and sludge wastes, are potential sources for biochar production and have been used widely to immobilize As species in water (Amen et al. 2020; Vithanage et al. 2017). Nevertheless, pristine biochar frequently exhibits a relatively low adsorption capability, because it depends predominantly on its surface charge and is influenced by the electrostatic repulsion between the negatively charged biochar surface and As oxyanions (Hu et al. 2015). Pristine biochar has not shown potential in As removal from aqueous solution, mainly because of its relatively low surface area and the effect of abiotic and/or biotic processes on the properties and As elimination capacity (Qiu et al. 2022).
The efficiency of different types of biochar for As removal from water has been widely investigated. Table 1 shows the As adsorption capacities of pristine biochars. Experiments using woody biomass-derived biochars to remove As(III) from water show that oak-bark char has significant adsorption potential (Mohan et al. 2007; Shaheen et al. 2019). In an aqueous solution, As(V) is adsorbed by sewage sludge (BC-SS), solid waste (BC-SW), and rice husk (BC-RH), with the maximum adsorption rates being 0.0680 mg g-1, 0.0549 mg g-1, and 0.0405 mg g-1, respectively (Agrafioti et al. 2014a). The efficacy of biochar derived from Japanese oak wood (OW-BC) for As(V) and As(III) removal from contaminative well water has also been determined; maximum removal rates of 84% and 81% were achieved through As(V)- and As(III)-OW-BC at pH 7 and 6, respectively (Niazi et al. 2018b). In contrast, biochar derived from rice husk is not suitable, as it has low efficiency for immobilizing As species (Zhong et al. 2019). Overall, pristine biochars have a relatively lower adsorption capacity for As in water than modified biochars.
2.2 Modified biochars
Surface-modified biochar has been shown to have an excellent capacity for removing As from water and has received widespread research attention (Shaheen et al. 2022a, 2022c; Tan et al. 2016). Table 2 shows the As adsorption capacities of modified biochars. In recent studies, techniques including pyrolysis, physical modifications, and chemical modifications have been used to produce composites that effectively remove As from water (Trakal et al. 2018; Zhang et al. 2016). Furthermore, multiple chemical and/or physical modifications have been investigated to improve the physicochemical properties of biochar to enhance As adsorption efficiency. These modifications include changing the surface charge and functional groups of biochar (Mo et al. 2018). Attention has focused on applying modification techniques (physical/chemical treatments and composites) to enhance the adsorption of As from water, and multiple methods have been employed for biochar surface modifications. These include strong acids, strong bases, metallic iron, metal oxides, nanoparticles, and metal/nanoparticle composites (Cuong et al. 2021; Hussain et al. 2020b; Lata et al. 2019; Singh et al. 2020).
Table 3 summarizes studies using acids and bases (H2SO4, H3PO4, KOH, and H2O2), metal ions and oxides [iron (Fe), manganese (Mn), bismuth (Bi), zinc (Zn), cerium (Ce), ferric oxide (Fe2O3), manganese dioxide (MnO2), manganese oxide (MnO), zinc oxide (ZnO), and copper oxide (CuO)], acid/bases and metals, metal oxides composites [Fe–Mn oxides, Ca–Fe oxides, Ce–Mn oxides, nickel (Ni)–Mn oxides, Fe–Mn–Ce oxides], and nanoparticles [nanoscale TiO2, zerovalent iron nanoparticles (nZVI)].
2.2.1 Importance of acids and bases
With respect to acid/base modifications, using modified black carbon to remove As(V) from contaminated water has shown that H2SO4 pretreatment improves adsorption (Borah et al. 2008). Potassium hydroxide (KOH) has been applied to activate the surface of municipal waste-sludge biochar, increasing As(V) adsorption approximately 1.3-fold over that of pristine biochar. This result could be attributed to electron donor–acceptor interactions with negatively charged As species, as well as proton exchange with newly developed functional groups (Jin et al. 2014). Modifications with KOH and H2O2 were found to increase the surface area of biochar, which, in turn, increased the adsorption of As(V) four- and five-fold, respectively (Wongrod et al. 2018). Raw cotton-stalk biochar modified with KOH and H3PO4 to remove As(V) from water has also been investigated. In this case, the removal efficiency of KOH-modified biochar was superior (90–99.5%) to that of H3PO4-modified (84–98%) and pristine (81–98%) biochars. KOH-modified biochar has a higher adsorption capacity because of its smaller particle size, larger surface area, and greater porosity compared with that of pristine and H3PO4-modified biochars (Hussain et al. 2020).
2.2.2 Importance of metal iron (zerovalent iron and iron ion)
The application of metal-iron biochars has achieved some degree of success in removing As from water. Biochar impregnated with Fe is an attractive low-cost adsorbent material with higher adsorption capacity than pristine biochar (Hu et al. 2015). The most successful method is a zerovalent iron (ZVI) coating, as Fe is a highly effective adsorbent for As in water. Biochar-supported ZVI can also adsorb As onto the Fe(0) particles, with efficacy increasing (23–95%) as a function of the Fe content (Zhou et al. 2014b). The ZVI-biochar complex effectively removes As from contaminated drinking water and has a higher adsorption potential for As than pristine biochar.
Using banana pith as a raw material, a Fe-modified biochar has been used to remove As from aqueous solutions. After Fe impregnation, the surface area (31.6 m2g-1) increased by almost eight times, leading to a relatively higher adsorption capacity for As(V) compared to raw biochar (Lata et al. 2019). Using rice straw as a raw material, a novel Fe-modified biochar produced by FeCl3 modification has been used for As(V) removal from aqueous solutions. Compared to raw biochar, the modified biochar increased As(V) removal ability, with adsorption maxima of 28.5 mg g-1 (Nguyen et al. 2019) and 26.9 mg g-1 (Nham et al. 2019), respectively. Using sludge as a raw material, biochar for the immobilization of As(V) was successfully prepared with different Fe contents at pyrolysis temperatures of 350 oC and 700 °C. These sludge biochars showed superior adsorption of As(V), with maximum adsorption capacities ranging from 60.2 to 90.2 mg g-1 (Yu et al. 2021). The increased adsorption was attributed to the ferric chloride (FeCl3) salt pyrolyzed with the biomass (Bakshi et al. 2018).
2.2.3 Importance of metal ion (zinc and bismuth)
The removal efficiencies for As(III) by raw pinecone-derived biochar and a Zn-loaded counterpart were 66% and 88% from aqueous solutions, respectively (Van Vinh et al. 2015). The reported adsorption capacity of ZnCl2-activated biochar for As(III) from pig manure is 27.7 mg g-1 (Xia et al. 2016), and the adsorption capacity of Bi-impregnated wheat-straw biochar for As(III) is 2.5 mg g-1 (Zhu et al. 2016). Bi-impregnated wheat-straw biochar has also been shown to effectively remove As(III) from water (Zhu et al. 2018). Accordingly, Fe-modified biochar is popular for high-efficiency As removal from aqueous solutions. The highest efficiency level achieved by this type of modified biochar is 90.2 mg g-1, which is higher than the uptake capacities reported for Zn-modified and Bi-modified biochar.
2.2.4 Importance of iron oxide
Biochar is increasingly considered as a supporting material for Fe oxide (Fe2O3) particles due to its stability and low cost (Cope et al. 2014; Hu et al. 2015). Fe oxides have a high affinity for As compounds and can remove various As species selectively (Chang et al. 2012; Jovanovic et al. 2011; Shaheen et al. 2022b). Composites of biochar impregnated with hematite (Fe2O3) show outstanding As adsorption ability from water compared with that of pristine biochar (Chen et al. 2011; Wang et al. 2015b; Zhang et al. 2013). The adsorption capacity of biochar modified with red mud (RM-BC) for As(V) reaches 5.92 mg g-1, approximately 10 times that of untreated biochar. Increased As(V) adsorption by RM-BC is attributed to the presence of Fe oxides (hematite and magnetite) in the mud (Wu et al. 2017). Accordingly, biochar modified with Fe oxide is considered an environmentally sustainable and effective adsorbent to replace raw biochar for treating As-contaminated groundwater and wastewater.
2.2.5 Importance of manganese dioxide, manganese oxide, zinc oxide, and copper oxide
Biochar loaded with MnO2 has been found to be a good adsorbent for As in groundwater and wastewater (Shaheen et al. 2022d). Corn-stalk biochar modified with MnO2 adsorbed 11.4–20.1 mg g-1 of As(III) from water (Yu et al. 2017). An MnO2-modified rice-husk biochar composite used to remove As(III) from groundwater achieved a ten-fold enhancement compared with that of pristine biochar (Cuong et al. 2021). The removal ability of MnO-modified biochar increases from 64% to 91% (approximately 34.1 mg/g) as a function of the proportion of an amorphous MnO coating (Trakal et al. 2018).
Impregnating corn-cob and coffee-husk biochars with ZnO has been shown to improve As(V) adsorption capacities and rates. The maximum equilibrium As(V) adsorption capacity of ZnO-impregnated corn-cob biochar is 25.9 mg g-1 (Cruz et al. 2020). Enhanced adsorption of As has also been demonstrated with a novel nanocomposite of Sesbania bispinosa biochar (SBC) with either MnO (SBC/MnO) or CuO (SBC/CuO) nanoparticles. The maximum As adsorption of the SBC/CuO composite is 12.5 mg/g while that of SBC/MnO and SBC is 7.34 mg g-1 and 7.33 mg g-1, respectively. Excellent stability and reusability have been observed using SBC/CuO (Imran et al. 2021). Accordingly, biochar-derived MnO2, MnO, ZnO, and CuO-impregnated biochar are considered promising As adsorbents.
2.2.6 Importance of acid/bases and metals
Magnetization of Fe2O3 and Fe3O4 particles (through Fe(II)/Fe(III)-NaOH) to biochars by chemical co-precipitation increases As adsorption from water (Dewage et al. 2018; Karunanayake et al. 2019). Chemical modification involves the use of acids, bases and/or strong oxidants such as KMnO4 and H2O2 with the aim of altering the functional groups on the surface of biochars (Chemerys and Baltrenaite 2018). An active MnO2/rice husk biochar composite (MBC) was prepared to enhance As(III) removal for groundwater remediation, for which pH was an important factor influencing the As(III) removal capacity. For example, under alkaline conditions (0.01 M NaOH), the As(III) and As(V) removal capacity of MBC was notably lower than under acidic (0.01 M HCl) and neutral conditions due to the negative effects of electrostatic repulsion. Importantly, a powerful transformation capability of As(III) via MBC has been described; namely, only 5.9% of As(III) remained in solution under neutral conditions (Cuong et al. 2021). Canola straw-based biomass and biochar adsorbents were electrochemically modified with Fe oxide and used to remove As(V) from water. In this case, the Fe oxide-modified biochar treated at pH 3 (0.1 M HCl) achieved a higher As(V) uptake (Benis et al. 2021). Accordingly, biochar modified by metal oxides can achieve higher As removal capacities under appropriate acid or alkaline conditions.
2.2.7 Importance of double/multi-metal oxide
Double/multi-metal-based biochars have attracted attention for their potential in removing As from solution. Fe–Mn impregnated biochar is prepared by Fe–Mn co-precipitation, achieving an As(V) adsorption capacity of 3.44 mg g-1 compared to 0.50 mg g-1 for the control (Wang et al. 2015a). Corn-stem biochar impregnated with Fe and Mn oxides exhibited high As adsorption of 8.25 mg g-1 (Lin et al. 2017) and 8.80 mg g-1(Lin et al. 2019), respectively, which was attributed to Fe and Mn oxides increasing the surface area. Moreover, the As(V) removal from aqueous solution using Ca- and Fe-modified rice-husk biochar has been studied. A higher As(V) removal capacity (> 95%) has been found for Ca–Fe-modified biochars compared with non-impregnated biochars (Agrafioti et al. 2014b). Magnetic biochar modified with Ca–Fe adsorbed 85% of As(III) from water (Wu et al. 2018b). A novel adsorbent has also been prepared by loading Ce and Mn oxides onto wheat-straw-modified biochar for As(V) removal from water, with a capacity of 108.9 mg g-1, which is significantly higher than that of the original biochar (Liang et al. 2020). As-contaminated water has also been remediated by Fe–Mn–Ce oxide-modified corn-stalk biochar composites, with a remedial capacity 3.27 times higher than that of the corresponding raw biochar (Liu et al. 2019b). Accordingly, biochar-derived double/multi-metal oxides are also considered promising As adsorbents.
2.2.8 Importance of nanoparticles
The use of different nanomaterials and particle sizes has been shown to increase As adsorption from water, attributed primarily to the unique chemical and physical properties of nanomaterials that significantly improve As adsorption (Liu et al. 2011; Zhang and Gao 2013; Zhou et al. 2014a). Combining biochar derived from raw corn-cobs waste with nanoscale TiO2 enhanced As adsorption from contaminated water, achieving a maximum As(V) adsorption capacity of 118 mg g-1 (Lun et al. 2019). Nanoscale ZVI-activated carbon increased both As(III) and As(V) adsorption, with maximum adsorption capacities of 18.2 mg g-1 and 12.0 mg g-1, respectively, at an equilibrium time of 72 h (Zhu et al. 2009). Biochar-based ZVI nanoparticles can be prepared from Cassia fistula pods through pyrolysis, with maximum As(III) and As(V) adsorption capacities of 1.04 mg g-1 and 1.40 mg g-1, respectively (Shaikh et al. 2020). Comparing different nanoparticles, nanoscale TiO2 supported by biochar has shown the highest efficacy (118 mg g-1) for removing As from contaminated water.
In view of this existing evidence, various modified biochars (acids and bases, metal iron and oxides, acid/bases and metals, double/multi-metal oxides composites, and nanoparticles) are considered promising adsorbents for As removal and show significant potential for the remediation of As-contaminated water. Currently, the most conventional adsorbent is Fe, while biochars loaded with nanoparticles show superior removal efficacy.
3 Biochars for the remediation of arsenic in contaminated soils
3.1 Soils remediation using pristine biochars
Biochar has been identified as an effective amendment that immobilizes As in soils (Chen et al. 2018; Garcia-Carmona et al. 2017). For instance, two ready-made materials prepared from biowastes (oyster shell waste and biochar) have been evaluated in highly contaminated (up to 15,000 mg kg-1 As) soils. Both oyster shell waste and biochar effectively decreased the leaching capacity of As in acidic soils, reducing the exchangeable As content from 105.8 to 54.0 mg kg-1, decreasing soluble As(V) from 119.8 to 56.4 mg L-1, and decreasing soluble As(III) from 374.9 to 185.9 mg L-1 (Chen et al. 2018). Wheat-straw biochar (Triticum aestivum) has been screened at different pyrolysis temperatures for removing As from soils. The As removal rate reached 83.7% after 60 min at 25 °C. The mechanisms for the adsorption and removal of As include chemisorption, physisorption, diffusion, and ion exchange (Kumar and Bhattacharya 2021). Less attention has been paid to the immobilization of As in soils, giving rise to a current research gap. Therefore, developing effective and affordable biochars for removing As from agricultural and industrial soils remains a major challenge.
3.2 Soils remediation using modified biochars
3.2.1 Importance of iron, iron oxide, and zerovalent iron nanoparticles
Altering the surface and structural properties of biochar to improve As removal in soils is a major remediation strategy (Beesley and Marmiroli 2011; Zhang et al. 2013, 2022b). Arsenic exists mainly in oxyanion form and has a high affinity for Fe iron; therefore, mixing ZVI with biochar is an attractive strategy for soils remediation. The application of ZVI and biochar effectively immobilizes As in paddy soils and decreases As content by 61% in rice grains (Qiao et al. 2018). Biochars modified with FeCl3 (Biochar–FeCl3), Fe-oxyhydroxy sulfate (biochar–FeOS), and ZVI (biochar–Fe) reduce the As content in soils by 11.0–28.4%, 14.0–30.4%, and 18.0–35.2%, respectively. Compared with other Fe-modified biochars, biochar–FeOS shows a superior immobilization effect and has the potential to remediate As-contaminated paddy soils (Wu et al. 2018a). Magnetic biochar modified with Fe oxide also improves the adsorption of As in soils, primarily attributable to the unique high adsorption capacity of Fe oxide with respect to As (Wu et al. 2018b). In order to study the practical application of Fe3O4-loaded magnetic biochar in contaminated soils, a dry magnetic separation technique was investigated to verify the feasibility of permanent removal As from soil. Dry magnetic separation results demonstrated that approximately 25% of total As was removed from the magnetic biochar amended soils, and As binding to poorly crystallized Fe compounds was the main retrieved fraction (Li et al. 2021a, b). To enhance the immobilization of As by pristine sawdust biochar, biochar and nZVI-embedded composites have been prepared and applied in two Chinese mining areas, Jinya County in Guangxi Province and Dayu County in Jiangxi Province. In the soils from both of these areas, nZVI-embedded biochar reduced labile As by more than 93% and bioaccessible As by more than 85% (Fan et al. 2020). Accordingly, Fe also has the most significant effect on the ability of biochar to remove As from soils.
3.2.2 Importance of manganese dioxide and manganese oxide
Both Mn oxide and Mn oxide-modified materials have high oxidation potentials and strong As immobilization abilities. Therefore, these materials show potential as cost-effective adsorbents for As in soils (Gregory et al. 2014; Yu et al. 2015). Mn oxide-modified biochar composites (MBCs) have been prepared by pyrolyzing permanganate (KMnO4) and biochar, and their As adsorption properties in red soils have been investigated. Mn oxides increased the hydrogen to carbon (H/C) ratio, oxygen to carbon (O/C) ratio, surface hydrophilicity, and surface adsorption capacity of biochars, indicating that Mn oxides play a vital role in As removal (Yu et al. 2015). Mn oxide-modified biochar remediated As-contaminated rice soils and reduced As bioaccumulation in the rice grains of plants growing in the contaminated soil (Yu et al. 2017). However, soils are more complex than water; soil is composed of various granular minerals, organic matter, water, air, and microorganisms. The material can be categorized into three parts—solid part, liquid, and gas parts, with the liquid part consisting mainly of water. Therefore, the As content of soil is mainly reduced by immobilization, while the As content of water is mainly reduced by adsorption. Thus, understanding the processes that influence the behavior of As in biochar-amended soils requires a comprehensive consideration of biochar type and soil properties optimize biochar selection. Further applied research is required to bridge this knowledge gap.
4 Factors influencing arsenic removal using pristine and modified biochars
Biochar adsorbs As in water and inhibits its mobilization in soils through different mechanisms, which are impacted by a range of factors. Numerous studies have addressed the specific factors that influence the immobilization potential of biochars, which can be abiotic [e.g. the preparation conditions of biochar (pyrolysis temperature), pH, dissolved organic carbon (DOC), phosphate, silicate, and sulfate] and biotic (microbial activities) in both water and soils (Fig. 2).
4.1 Abiotic factors
4.1.1 Preparation conditions of biochar-pyrolysis temperature
Biochar surfaces contain microporous to mesoporous structures, with different surface functional groups, including hydroxyl (–OH), carboxyl (–COOH), carbonyl (C=O), lactone, and alcohol groups, as well as inorganic mineral species and ions (e.g., CaCO3, PO43–). High adsorption capacities depend on a high specific surface area, and the surface area of biochar is affected by the pyrolysis temperature (Fang et al. 2014; Inyang et al. 2016; Niazi et al. 2018a; Zhang et al. 2016). A higher biochar yield is achieved at lower temperatures, whereas greater porosity is achieved at higher temperatures (Siddiq et al. 2022). Biochar prepared at low temperatures contains oxygenated functional carboxylic and phenolic groups (Tan et al. 2015), and biochar synthesized at low temperatures (200–400 °C) is preferred for As removal because it has higher O/C molar ratios (Uchimiya et al. 2011) that allow As to bind to its O-containing functional groups (Ahmad et al. 2014). The adsorption efficiency of two types of algal-derived Fe-biochars was shown to decrease as a function of increased pyrolysis temperature. The optimal Fe-biochar for each biomass, with a biosorption capacity of 62.5–80.7 mg g-1, was prepared by slow pyrolysis at 300 °C (Johansson et al. 2016). Accordingly, a relatively low pyrolysis temperature can increase the As adsorption efficiency of the resulting biochar compared with that of biochar produced at higher temperatures.
4.1.2 Influential factors in the adsorption process
4.1.2.1 pH
pH is a key factor in the removal capacity of As(V) and As(III). The parameter pHPZC (pH of the point of zero charge) is used to estimate the development of net charge on the biochar surface as a function of pH. Biochar surfaces carry both negative and positive charges, with the surface above pHPZC being net negative and that below pHPZC net positive (Vithanage et al. 2017). Arsenic complexation with biochar functional groups regulates the adsorption of As; the As complexation behavior of carbonyl, functional hydroxyl, and amino groups changes as a function of the solution pH. For example, an increase in pH leads to the deprotonation of the carbonyl group, and the carbonyl group can effectively bind with positively charged As species. When biochar interacts with an As solution, As is attracted to the biochar because of the presence of > ROH– and > RCOO– surface species. After first-order chemisorption, biochar and As molecules form complex structures (Yang et al. 2022a; Yuan et al. 2011; Zhang and Gao 2013).
The As(V) and As(III) removal capacities of an activated MnO2/rice-biochar composite under alkaline conditions were lower than those under neutral and acidic conditions, ascribed to the adverse effect of electrostatic repulsion (Cuong et al. 2021). Using raw pinecone and Zn-loaded modified biochars to remove As(III) from aqueous solutions has also been investigated. In the acidic (2–4) pH range, the removal of As(III) was comparable (66.1% and 87.6%), while in the 4–12 pH range, removal decreased because the surface became increasingly negative, causing the repulsion of negatively charged aqueous As species (Van Vinh et al. 2015). In addition, As(V) removal by canola-straw-based biochar adsorbents from water through electrochemical modification has been explored. It was found that biochar treated at a lower pH of 3 had higher As(V) adsorption capacity (Benis et al. 2021). Accordingly, pH can change the adsorption capacity of As by changing the functional groups and charge on the surface of biochar.
The application of biochar usually increases the pH of soils because it contains a substantial amount of ash. High pH can reduce the positive charge of the soil-biochar system, reducing the adsorption or precipitation of anionic As (Liang et al. 2006). Alkaline pH has been shown to increase the mobility of As because oxyanionic As species in soils undergo ion exchange with hydroxyl ions (Shin et al. 2016). However, small changes in the pH may not affect As adsorption in biochar-modified soils (Nagodavithane et al. 2014). Currently, relatively little research exists beyond the knowledge that acidic pH in water and soils facilitates biochar-based As adsorption.
4.1.2.2 Dissolved organic carbon
Dissolved organic carbon (DOC) is a key factor in the remediation of As-contaminated soils by biochar, as it affects the migration of As independent of soils or biochar types (Fig. 2) (Kim et al. 2018). Not only is DOC the most mobile organic ligand in soils, but negatively charged DOC has also been shown to enhance desorption of As through competition (Beesley et al. 2014), with adsorption onto the surface of Fe oxide in competition with oxyanions, and also increases the migration of oxyanions in soils (Jeon et al. 2018). However, the possibility of As complexing with DOC in biochar is particularly important in the context of soils with a high organic carbon content (Hartley et al. 2009; Nagodavithane et al. 2014). The competition between DOC and As for adsorption sites on soil surfaces can lead to increased concentrations of aqueous As (Beesley et al. 2014). The effects of DOC and As interactions on soil-As interactions need to be studied in more detail at the molecular level.
4.1.2.3 Phosphate, silicate, and sulfate
The phosphate content in soils is enhanced more than 20-fold by the addition of biochar (Beesley et al. 2013; Xu et al. 2013). Coexisting phosphate anions, with chemical properties similar to those of As, enhance the desorption of As from soils through competitive adsorption (Hartley et al. 2009) and are associated with As bioavailability and mobility (Meharg and Hartley-Whitaker 2002). Several factors explain the influence of coexisting ions, in particular ionic speciation and ionic radius (Wang et al. 2018). For example, PO43– significantly reduces As(V) adsorption by Fe-impregnated biochar (Baig et al. 2014), because AsO43– and PO43– have similar ionic structures, enabling them to compete for the same adsorption sites (Hu et al. 2015) (Fig. 2). Silicate significantly inhibits the adsorption of As(V), as these two species have different inner-sphere complexation mechanisms (Gao et al. 2013). In addition, the presence of sulfate ions slightly inhibits As adsorption by Fe-loaded biochar because of the differing external outer-sphere complexation mechanisms between the coexisting ions and sulfate formation (Wei et al. 2019b). Sulfate ions may also compete with As oxyanions for adsorption to Fe(hydro)oxides (Fu et al. 2017; Yang et al. 2022a). Accordingly, phosphate, silicate, and sulfate affect both adsorption and desorption of As through different mechanisms, such as competition and inner-sphere and outer-sphere surface complexation.
4.2 Biotic factors: microbial activities
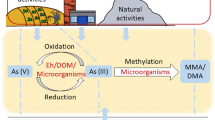
Few studies have investigated the influence of microorganisms on biochar soil remediation (Chen et al. 2016; Gregory et al. 2014, 2015; Lehmann et al. 2015). The focus of most studies is the biological effects of biochar application on changes in As mobility. Biochar amendment has been shown to increase soil microbial activity (Zhang et al. 2014), which can be attributed to the nutrients and bioavailable C components that biochar provides. This provides substrates for the microbial metabolism, promoting increased activity of soil microorganisms, which increases As cycling and mobility in the soils (Gregory et al. 2014). Furthermore, soil microbial activity further affects As speciation and DOM type and distribution. The reduction of As(V) to As(III) by biochar in As(V)-contaminated tailings sediments has been explored, and As(V)-reducing bacteria such as Anaeromyxobacter, Geobacter, Pedobacter, and Desulfosporosinus have been shown to increase after biochar addition. Dissolved organic matter concentrations increase in biochar-modified mine sediments, influencing the mobility and bioavailability of As (Chen et al. 2016) (Fig. 2). Accordingly, multiple abiotic and biological factors affect the adsorption efficiency of biochar, leading to significant changes in its adsorption capacity. Future studies should comprehensively investigate the influences of multiple factors on biochar remediation in both water and soils, particularly in complex soils environments in which multiple abiotic and biological factors will play important roles in As immobilization.
5 Mechanisms of arsenic adsorption/immobilization by pristine and modified biochar
5.1 Chemical mechanisms
There are different mechanisms for the immobilization of As(III) and As(V) by biochar owing to the numerous surface functional groups on the biochar surface. Figure 3 illustrates the principal mechanisms of As absorption from water by pristine and modified biochars, and Table 3 shows the modifiers and their mechanisms. The adsorption of As(III) and As(V) onto the surface of pristine biochar is limited owing to the negative charge and low potential for anion exchange of most biochars. Therefore, biochar requires modification for enhanced removal of As(III) and As(V) from water and soils. Newly introduced biochar surface modifications provide an enhanced affinity for targeted pollutants, which includes the formation of surface complexes by strong intermolecular interactions (Rajapaksha et al. 2016). Surface complexation/precipitation, oxidation and reduction, electrostatic interactions, ion exchange, and enhancing of surface functional groups are the major mechanisms that trigger As adsorption onto biochar surfaces (Tan et al. 2016; Zama et al. 2017; Zhang et al. 2016). Increasingly, investigations have focused on enhancing biochar capacity by the extensive use of acids or bases, metal ions or oxides, such as Fe, hydrous ferrous oxide (FeO), MnO2, and Fe–Mn oxides.
5.1.1 Importance of acid and bases
Arsenic adsorption relies on the presence of functional groups on the surface of biochar. Firstly, H2SO4 has been used to enhance acidic functional groups (Kastner et al. 2012). Secondly, the porous structure and adsorption properties of biochars have been enhanced using KOH (Regmi et al. 2012). By separating the carbon layer from the surface of biochar, K2O and K2CO3 formed by KOH increases biochar porosity and surface area (Diaz-Teran et al. 2001). After KOH modification, the specific porous structure and surface area of biochar also improved significantly, and the functional group densities and surface charge increased, leading to higher affinity for As(V); in turn, this leads to enhanced As(V) removal from aqueous solution (Jin et al. 2014). Thirdly, hydrothermal carbonization of peanut-hull biochar with H2O2 oxidized the surface and increased the proportion of oxygen-containing functional groups (Xue et al. 2012). Fourthly, KOH- and H2O2-modified biochar have inverse As(III) and As(V) adsorption capacities; the As(V) adsorption capacities of KOH- and H2O2-modified biochar increased five- and four-fold, respectively, which was attributed to an increased surface area (Wongrod et al. 2018). In contrast to As(V), the As(III) adsorption capacity of biochar after KOH and H2O2 treatments decreased by 72% and 38%, respectively. KOH dissolves the ash content of biochar, whereas H2O2 oxidizes its organic matter content, thereby negatively affecting adsorption (Wongrod et al. 2019). Accordingly, KOH- and H2O2-modified biochar increase As(V) removal from but decrease As(III) removal from aqueous solution.
5.1.2 Importance of iron
The adsorption mechanism between Fe and As mainly involves anion exchange, inner-sphere complexation, strong electrostatic attraction, co-precipitation, and diffusion within particles. The main mechanism of As removal by Fe-modified biochar is anion exchange between As in the solution and –OH in the adsorbent structure (Deschamps et al. 2005). Firstly, in Fe-modified biochar, As(V) or As(III) create strong inner-sphere complexes with Fe, which explains its superior As removal performance. The original Fe–OH ligands are broken to form different Fe–O–As ligands during As–Fe complex formation (Mukherjee et al. 2011). Secondly, the elevated adsorption of As(V) by Fe(III)-modified biochar has been attributed to the strong electrostatic attractions between the anionic As species (H2AsO4– and HAsO42–) and positively charged FeOH2+ on the surface of the modified biochar (pHPZC = 9.73) (Jimenez-Cedillo et al. 2013). Thirdly, the adsorption of As on the surface of preexisting FeOOH minerals and the diffusion within the particles (Bakshi et al. 2018) could play a significant role in As removal from solution. Fourthly, As(V) is converted to As(III), Fe(II) is converted to Fe(III), and a strong bond forms between As and the oxygen-containing functional groups of Fe-impregnated biochar (Hu et al. 2015). Simultaneously, As(V) is reduced to As(III), ZVI is oxidized to Fe(III), and then As(III) and Fe(III) co-precipitate, forming Fe(As)OOH on the biochar surface (Bakshi et al. 2018). Notably, co-precipitation is faster than intraparticle diffusion and surface adsorption (Bakshi et al. 2018).
5.1.3 Importance of iron oxide
Adsorption between FeO and As primarily involves a redox reaction between Fe(II) and As(V), and the presence of mesopores and elevated specific surface area, which lead to a shortening of the diffusion distance of As and the maximization of Fe utilization, electrostatic interactions, and the formation of bidentate (≡Fe–O)2AsOH and monodentate ≡Fe–OAs(OH)2 complexes. The presence of FeO on the biochar surface facilitates As(V) adsorption and removal, controlled by the redox reaction between Fe(II) and As(V) (Agrafioti et al. 2014a; Shaheen et al. 2022b). At neutral pH conditions, FeO has abundant adsorption sites, a large specific surface area, a net positive surface charge, and a strong As adsorption capacity (Guo et al. 2013; Li et al. 2017). Fe oxide-modified rice husks provide surface chemistry conducive to As adsorption, with the modified mesoporous volume and specific surface area for As being superior to those of Fe particles alone. The importance of the media pore size has been demonstrated in Fe oxide amendment and As adsorption (Cope et al. 2014). The vertical growth of Fe oxide on biochar surfaces improves adsorption by shortening the diffusion distance of As and maximizing Fe utilization (Wei et al. 2019b). Compared with pristine biochar, hematite-modified biochar shows increased ability to remove aqueous As, possibly because c-Fe2O3 particles act as adsorption sites on the carbon surface through electrostatic interactions (Wang et al. 2015b). The primary surface Fe oxide on Fe-loaded rice-husk and wheat-husk biochars is Fe3O4. These adsorbents chemisorb As(III) with surface ≡Fe–OH functional groups by forming bidentate (≡Fe–O)2AsOH and monodentate ≡Fe–OAs(OH)2 complexes (Singh et al. 2020).
5.1.4 Importance of manganese oxide
Mn oxides are used widely as oxidants to remove As(III). The process is characterized by the progressive reduction of Mn(IV) to Mn(II), which could be explained by the specific adsorption of As to surface hydroxyl groups, co-precipitation, and/or ion exchange (Scott and Morgan 1995). Biochar treated with MnO2 is more positively charged, increasing As(III) attraction and oxidation, and promoting the adsorption of As(V) (Villalobos et al. 2014; Ying et al. 2012). MnO2/rice-husk modified biochar composites have been used to remove As(III) from groundwater, and their performance has been attributed mainly to As(III) redox transformations by MnO2, which increases their efficiency for removing As(V). The As(III) oxidation and removal mechanisms of As(V) and As(III) by MnO2/biochar include the following: (1) As(III) is adsorbed partially on the surface, and (2) As(III) oxidation to As(V) occurs simultaneously with Mn(IV) reduction to Mn(III) and Mn(II). Furthermore, As(V) and Mn(II) form MnHAsO4·H2O precipitates on the MBC-100 surface (Cuong et al. 2021). The impregnation of biochar with MnOx was shown to improve the functional group distribution, surface morphology, and elemental composition of biochar, which increased the As sorption capacity (Shaheen et al. 2022c).
5.1.5 Importance of iron-manganese oxide
An Fe–Mn binary oxide adsorbent with effective As removal capabilities has been developed. Binary sorbents demonstrate two main benefits for treating water and soil contaminated with As(V) or As(III) compared with pure Fe and Mn oxides. First, conventionally, Fe–Mn oxides are used to remove As from water through a mechanism dependent on As(III) oxidation to As(V) (Lin et al. 2017). This improvement is mainly attributed to the presence of two metal oxides (Fe2O3 and MnO) and the bimetal oxide MnFe2O4 (Hu et al. 2015). The Fe–Mn oxide particles increase the oxidation of As(III) to As(V) in the outer sphere. Based on the high adsorption capacity of Fe–Mn oxide-impregnated biochar, a method for surface adsorption/oxidation-based removal of As has been proposed, indicating its suitability for efficient water decontamination (Lin et al. 2019). KMnO4–Fe(II) biochar has been used to catalyze As removal from water. Fe–Mn oxide biochar has a higher specific surface area and oxidizes As(III) to As(V), with the material having high adsorption capacity for As (Ai et al. 2019).
In conclusion, increased As fixation on modified biochar surfaces is induced by surface complexation, ion exchange, co-precipitation, oxidation and reduction, and electrostatic interactions. Multiphase biochar composites function through multiple mechanisms, ultimately allowing the biochar to absorb As more efficiently by optimization of the biochar pore structure and the presence of surface functionalities that promote adsorption and/or redox transformations of As. Moreover, the mechanisms of As adsorption by acid, base, iron oxide, and manganese oxide modified biochar are relatively well-known, while those of biochars modified with other metals or nanomaterials remain understudied, and the mechanisms and relationships of As adsorption in the co-presence of cations and anions in solution (i.e., competition for adsorption) are also not well understand. Accordingly, these aspects require further investigation.
5.2 Microbial mechanisms
Relatively few studies have been conducted on the roles of reactions catalyzed by microorganisms in As remediation by biochar in soils (Bandara et al. 2020; Chen et al. 2016; Gregory et al. 2014, 2015; Lehmann et al. 2015). Microorganisms are abundant in agricultural soils, with many being genetically resistant to As. Moreover, many species can survive extreme As stress and possess genes that promote their survival in As-contaminated soils (Anantharaman et al. 2016; Serna-Chavez et al. 2013). Microorganisms play a significant role in controlling As reduction and oxidation reactions in the natural environment. Therefore, inoculating modified biochar with bacteria has the potential to enhance the remediation of As-contaminated water and soils. The interactions between microorganisms and different As species are important factors to consider (Huang 2014). Studies have been conducted on the interactions between several microbial species and As, and the mechanisms of As transformation to lower and higher solubility As species have been elucidated. After adding biochar, As(V)-reducing Anaeromyxobacter, Pedobacter, Geobacter, and Desulfosporosinus bacterial populations were observed to increase. This biochar-bacterial consortium promoted the reduction of As(V) to As(III) in biochar-modified mine sediments, thereby influencing both As mobility and bioavailability (Chen et al. 2016).
Furthermore, microorganisms can detoxify As. The surface adsorption of As, oxidation or reduction of inorganic As, methylation and demethylation of As, and chelation of cysteine-rich polypeptides are the main mechanisms of As detoxification. The transfer of genes from As-resistant microorganisms to other microbial species shows potential for enhancing bioremediation (Valls and de Lorenzo 2002). Such detoxification is influenced by As speciation, microbial composition, and the potential interactions between microorganisms and nutrients. For instance, when the As concentration is > 1 mg L-1, pure microbial strains, such as Dunaliella tertiolecta and MLH-1 are poisoned (Duncan et al. 2013; Oremland et al. 2004). Inserting the gene encoding As(III) S-adenosyl methionine methyltransferase (arsM) into Bacillus idriensis and Sphingomonas desiccabilis has been shown to result in a tenfold increase in methylated As gas, enabling As to evaporate from soils (Gupta and Singh 2017). Microorganisms in genera such as Achromobacter, Bacillus, Ochrobactrum, Stenotrophomonas, Pseudomonas, Brevundimonas, Comamonas, Microbacterium, and Sinorhizobium can decrease toxicity by increasing As mobility and improving the bioavailability of As to plants (Plewniak et al. 2018).
Nevertheless, biosafety concerns, including the possibility of horizontal gene transfer, must be considered before adding these microorganisms into sensitive environments. Accordingly, further study is necessary to facilitate in-depth understanding of mechanisms at the molecular level, regardless of the success of chemical and microbiological reactions in immobilizing As. Elucidating the molecular interactions that occur during biochar adsorption of As is of theoretical and practical significance.
6 Challenges and perspectives
Arsenic contamination of water and soils poses a significant threat to humans, particularly in Southeast and South Asia. Treating As-contaminated water and soils with biochar has the potential to reduce As contamination and prevent its adverse effects on human health. Several studies have been published on biochar remediation of As-contaminated water and soils systems. This review has summarized recent developments in pristine and modified biochar applications for the treatment of contaminated water and soils. Pristine and modified biochars are complex materials, with chemical and physical characteristics that vary significantly based on the raw materials and the conditions of their pyrolysis and subsequent modifications. Improved understanding of these properties is required to optimize As adsorption capacity.
Although the application of biochar as an adsorbent is increasing, some research gaps remain and the following research directions should be considered: (1) more studies are required for improving biochar adsorption using a combination of multiple metals and nanomaterials. Nanoparticles are one such option because of their high reactivity and ease of separation from soils; (2) the surface functionalization of biochar at the molecular level requires further study. For example, amending amide functional groups to the biochar surface could contribute significantly to As adsorption, and studies could provide clarity regarding the relevant mechanism involved; (3) few studies have focused on organic As species, such as methylated As and thio-As(V) compounds. Biochar provides living space for microorganisms; therefore, bacteria combined with biochar could effectively promote the biotransformation of methylated As, particularly the generation of gaseous methyl As. The gasification of As by biochar could also be a future research direction; and (4) actual water and soil substrates are more complex than general laboratory solutions containing only As and adsorbents. Future research should, therefore, preferably use water from field sites, wastewater, and soils rich in As and co-occurring ions for improved evaluation of real-world adsorption behavior.
Future research should aim to expand the use of modified biochar for the rehabilitation of systems contaminated by various As species. Further optimization and economic feasibility analyses are required before successful commercial and industrial applications could be launched, which would pave the way for the sustainable mitigation of As pollution at a global scale. However, from the perspective of environmental protection and sustainable development, selecting the most appropriate pristine biochar to remove As from water and soils remains a challenging task. Although remarkable progress has been achieved in using biochar to treat contaminated soil and water, a significant need remains for further research.
Availability of data and materials
The datasets analyzed in the current study are available from the corresponding author on reasonable request.
References
Aftabtalab A, Rinklebe J, Shaheen SM, Niazi NK, Moreno-Jimenez E, Schaller J, Knorr KH (2022) Review on the interactions of arsenic, iron (oxy)(hydr)oxides, and dissolved organic matter in soils, sediments, and groundwater in a ternary system. Chemosphere 286:131790
Agrafioti E, Kalderis D, Diamadopoulos E (2014a) Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J Environ Manage 133:309–314
Agrafioti E, Kalderis D, Diamadopoulos E (2014b) Ca and Fe modified biochars as adsorbents of arsenic and chromium in aqueous solutions. J Environ Manage 146:444–450
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Ai J, Zhang WJ, Chen FF, Liao GY, Li DD, Hua X, Wang DS, Ma T (2019) Catalytic pyrolysis coupling to enhanced dewatering of waste activated sludge using KMnO4-Fe(II) conditioning for preparing multi-functional material to treat groundwater containing combined pollutants. Water Res 158:424–437
Alam MA, Shaikh WA, Alam MO, Bhattacharya T, Chakraborty S, Show B, Saha I (2018a) Adsorption of As(III) and As(V) from aqueous solution by modified Cassia fistula (golden shower) biochar. Appl Water Sci 8(7):198
Alam MS, Gorman-Lewis D, Chen N, Flynn SL, Ok YS, Konhauser KO, Alessi DS (2018b) Thermodynamic analysis of nickel(II) and zinc(II) adsorption to biochar. Environ Sci Technol 52(11):6246–6255
Ali S, Rizwan M, Shakoor MB, Jilani A, Anjum R (2020) High sorption efficiency for As(III) and As(V) from aqueous solutions using novel almond shell biochar. Chemosphere 243:125330
Alchouron J, Navarathna C, Chludil HD, Dewage NB, Perez F, Hassan E, Pittman CU, Vega AS, Mlsna TE (2020) Assessing South American Guadua chacoensis bamboo biochar and Fe3O4 nanoparticle dispersed analogues for aqueous arsenic(V) remediation. Sci Total Environ 706:135943
Alkurdi SSA, Herath I, Bundschuh J, Al-Juboori RA, Vithanage M, Mohan D (2019) Biochar versus bone char for a sustainable inorganic arsenic mitigation in water: what needs to be done in future research? Environ Int 127:52–69
Amen R, Bashir H, Bibi I, Shaheen SM, Niazi NK, Shahid M, Hussain MM, Antoniadis V, Shakoor MB, Al-Solaimani SG, Wang HL, Bundschuh J, Rinklebe J (2020) A critical review on arsenic removal from water using biochar-based sorbents: the significance of modification and redox reactions. Chem Eng J 396:125195
Anantharaman K, Brown CT, Hug LA, Sharon I, Castelle CJ, Probst AJ, Thomas BC, Singh A, Wilkins MJ, Karaoz U, Brodie EL, Williams KH, Hubbard SS, Banfield JF (2016) Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system. Nat Commun 7:13219
Baig SA, Zhu J, Muhammad N, Sheng TT, Xu XH (2014) Effect of synthesis methods on magnetic Kans grass biochar for enhanced As(III, V) adsorption from aqueous solutions. Biomass Bioenerg 71:299–310
Bakshi S, Banik C, Rathke SJ, Laird DA (2018) Arsenic sorption on zero-valent iron-biochar complexes. Water Res 137:153–163
Bandara T, Franks A, Xu JM, Bolan N, Wang HL, Tang CX (2020) Chemical and biological immobilization mechanisms of potentially toxic elements in biochar-amended soils. Crit Rev Environ Sci Technol 50(9):903–978
Beesley L, Marmiroli M (2011) The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ Pollut 159(2):474–480
Beesley L, Marmiroli M, Pagano L, Pigoni V, Fellet G, Fresno T, Vamerali T, Bandiera M, Marmiroli N (2013) Biochar addition to an arsenic contaminated soil increases arsenic concentrations in the pore water but reduces uptake to tomato plants (Solanum lycopersicum L.). Sci Total Environ 454:598–603
Beesley L, Inneh OS, Norton GJ, Moreno-Jimenez E, Pardo T, Clemente R, Dawson JJC (2014) Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ Pollut 186:195–202
Beiyuan J, Awad YM, Beckers F, Tsang DCW, Ok YS, Rinklebe J (2017) Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere 178:110–118
Benis KZ, Damuchali AM, Soltan J, McPhedran KN (2020) Treatment of aqueous arsenic—a review of biochar modification methods. Sci Total Environ 739:139750
Benis KZ, Soltan J, McPhedran KN (2021) Electrochemically modified adsorbents for treatment of aqueous arsenic: pore diffusion in modified biomass vs biochar. Chem Eng J 423:130061
Bolan N, Hoang SA, Beiyuan JZ, Gupta S, Hou DY, Karakoti A, Joseph S, Jung S, Kim KH, Kirkham MB, Kua HW, Kumar M, Kwon EE, Ok YS, Perera V, Rinklebe J, Shaheen SM, Sarkar B, Sarmah AK, Singh BP, Singh G, Tsang DCW, Vikrant K, Vithanage M, Vinu A, Wang HL, Wijesekara H, Yan YB, Younis SA, Van Zwieten L (2022) Multifunctional applications of biochar beyond carbon storage. Int Mater Rev 67(2):150–200
Borah D, Satokawa S, Kato S, Kojima T (2008) Surface-modified carbon black for As(V) removal. J Colloid Interface Sci 319(1):53–62
Brickson BE (2003) Field kits fail to provide accurate measure of arsenic in groundwater. Environ Sci Technol 37(1):35a–38a
Bundschuh J, Armienta MA, Morales-Simfors N, Alam MA, Lopez DL, Quezada VD, Dietrich S, Schneider J, Tapia J, Sracek O, Castillo E, Parra LMM, Espinoza MA, Guilherme LRG, Sosa NN, Niazi NK, Tomaszewska B, Allende KL, Bieger K, Alonso DL, Brandao PFB, Bhattacharya P, Litter MI, Ahmad A (2021) Arsenic in Latin America: new findings on source, mobilization and mobility in human environments in 20 countries based on decadal research 2010–2020. Crit Rev Environ Sci Technol 51(16):1727–1865
Cai CY, Zhao MH, Yu Z, Rong HW, Zhang CS (2019) Utilization of nanomaterials for in-situ remediation of heavy metal(loid) contaminated sediments: a review. Sci Total Environ 662:205–217
Chang QG, Lin W, Ying WC (2012) Impacts of amount of impregnated iron in granular activated carbon on arsenate adsorption capacities and kinetics. Water Environ Res 84(6):514–520
Chemerys V, Baltrenaite E (2018) A review of lignocellulosic biochar modification towards enhanced biochar selectivity and adsorption capacity of potentially toxic elements. Ukrain J Ecol 8(1):21–32
Chen BL, Chen ZM, Lv SF (2011) A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Biores Technol 102(2):716–723
Chen Z, Wang YP, Xia D, Jiang XL, Fu D, Shen L, Wang HT, Li QB (2016) Enhanced bioreduction of iron and arsenic in sediment by biochar amendment influencing microbial community composition and dissolved organic matter content and composition. J Hazard Mater 311:20–29
Chen YS, Xu JH, Lv ZY, Xie RJ, Huang LM, Jiang JP (2018) Impacts of biochar and oyster shells waste on the immobilization of arsenic in highly contaminated soils. J Environ Manage 217:646–653
Cope CO, Webster DS, Sabatini DA (2014) Arsenate adsorption onto iron oxide amended rice husk char. Sci Total Environ 488:558–565
Cruz GJF, Mondal D, Rimaycuna J, Soukup K, Gomez MM, Solis JL, Lang J (2020) Agrowaste derived biochars impregnated with ZnO for removal of arsenic and lead in water. J Environ Chem Eng 8(3):103800
Cuong DV, Wu PC, Chen LI, Hou CH (2021) Active MnO2/biochar composite for efficient As(III) removal: Insight into the mechanisms of redox transformation and adsorption. Water Res 188:116495
Cuong DV, Wu PC, Liou SYH, Hou CH (2022) An integrated active biochar filter and capacitive deionization system for high-performance removal of arsenic from groundwater. J Hazard Mater 423:127084
Deschamps E, Ciminelli VST, Holl WH (2005) Removal of As(III) and As(V) from water using a natural Fe and Mn enriched sample. Water Res 39(20):5212–5220
Dewage NB, Liyanage AS, Pittman CU, Mohan D, Mlsna T (2018) Fast nitrate and fluoride adsorption and magnetic separation from water on alpha-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar. Biores Technol 263:258–265
Diaz-Teran J, Nevskaia DM, Lopez-Peinado AJ, Jerez A (2001) Porosity and adsorption properties of an activated charcoal. Colloids Surf A Physicochem Eng Aspects 187:167–175
Duncan EG, Maher WA, Foster SD, Krikowa F (2013) The influence of arsenate and phosphate exposure on arsenic uptake, metabolism and species formation in the marine phytoplankton Dunaliella tertiolecta. Mar Chem 157:78–85
El-Naggar A, El-Naggar AH, Shaheen SM, Sarkar B, Chang SX, Tsang DCW, Rinklebe J, Ok YS (2019a) Biochar composition-dependent impacts on soil nutrient release, carbon mineralization, and potential environmental risk: a review. J Environ Manage 241:458–467
El-Naggar A, Lee SS, Rinklebe J, Farooq M, Song H, Sarmah AK, Zimmerman AR, Ahmad M, Shaheen SM, Ok YS (2019b) Biochar application to low fertility soils: a review of current status, and future prospects. Geoderma 337:536–554
Essandoh M, Kunwar B, Pittman CU, Mohan D, Mlsna T (2015) Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem Eng J 265:219–227
Fan CS, Tseng SC, Li KC, Hou CH (2016) Electro-removal of arsenic(III) and arsenic(V) from aqueous solutions by capacitive deionization. J Hazard Mater 312:208–215
Fan J, Chen X, Xu ZB, Xu XY, Zhao L, Qiu H, Cao XD (2020) One-pot synthesis of nZVI-embedded biochar for remediation of two mining arsenic-contaminated soils: arsenic immobilization associated with iron transformation. J Hazard Mater 398:122901
Fang QL, Chen BL, Lin YJ, Guan YT (2014) Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups. Environ Sci Technol 48(1):279–288
Foster SA, Pennino MJ, Compton JE, Leibowitz SG, Kile ML (2019) Arsenic drinking water violations decreased across the United States following revision of the maximum contaminant level. Environ Sci Technol 53(19):11478–11485
Fristak V, Moreno-Jimenez E, Fresno T, Diaz E (2018) Effect of physical and chemical activation on arsenic sorption separation by grape seeds-derived biochar. Separations 5(4):59
Fu DD, He ZQ, Su SS, Xu B, Liu YL, Zhao YP (2017) Fabrication of alpha-FeOOH decorated graphene oxide-carbon nanotubes aerogel and its application in adsorption of arsenic species. J Colloid Interface Sci 505:105–114
Gao XD, Root RA, Farrell J, Ela W, Chorover J (2013) Effect of silicic acid on arsenate and arsenite retention mechanisms on 6-L ferrihydrite: a spectroscopic and batch adsorption approach. Appl Geochem 38:110–120
Garcia-Carmona M, Romero-Freire A, Aragon MS, Garzon FJM, Peinado FJM (2017) Evaluation of remediation techniques in soils affected by residual contamination with heavy metals and arsenic. J Environ Manage 191:228–236
Gregory SJ, Anderson CWN, Arbestain MC, McManus MT (2014) Response of plant and soil microbes to biochar amendment of an arsenic-contaminated soil. Agric Ecosyst Environ 191:133–141
Gregory SJ, Anderson CWN, Camps-Arbestain M, Biggs PJ, Ganley ARD, O’Sullivan JM, McManus MT (2015) Biochar in co-contaminated soil manipulates arsenic solubility and microbiological community structure, and promotes organochlorine degradation. PLoS ONE 10(4):1–18
Guo HM, Ren Y, Liu Q, Zhao K, Li Y (2013) Enhancement of arsenic adsorption during mineral transformation from siderite to goethite: mechanism and application. Environ Sci Technol 47(2):1009–1016
Gupta S, Singh D (2017) Role of genetically modified microorganisms in heavy metal bioremediation. In: Advances in environmental biotechnology. Springer Nature Singapore, pp 197–214
Hartley W, Dickinson NM, Riby P, Lepp NW (2009) Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ Pollut 157(10):2654–2662
He RZ, Peng ZY, Lyu HH, Huang H, Nan Q, Tang JC (2018) Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal. Sci Total Environ 612:1177–1186
Hu X, Ding ZH, Zimmerman AR, Wang SS, Gao B (2015) Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res 68:206–216
Huang JH (2014) Impact of microorganisms on arsenic biogeochemistry: a review. Water Air Soil Pollut 225(2):1848
Hussain M, Imran M, Abbas G, Shahid M, Iqbal M, Naeem MA, Murtaza B, Amjad M, Shah NS, Khan ZU, Ul Islam A (2020) A new biochar from cotton stalks for As(V) removal from aqueous solutions: its improvement with H3PO4 and KOH. Environ Geochem Health 42(8):2519–2534
Hussain MM, Wang JX, Bibi I, Shahid M, Niazi NK, Iqbal J, Mian IA, Shaheen SM, Bashir S, Shah NS, Hina K, Rinklebe J (2021) Arsenic speciation and biotransformation pathways in the aquatic ecosystem: the significance of algae. J Hazard Mater 403:124027
Igalavithana AD, Kim KH, Jung JM, Heo HS, Kwon EE, Tack FMG, Tsang DCW, Jeon YJ, Ok YS (2019) Effect of biochars pyrolyzed in N-2 and CO2, and feedstock on microbial community in metal(loid)s contaminated soils. Environ Int 126:791–801
Imran M, Iqbal MM, Iqbal J, Shah NS, Khan ZU, Murtaza B, Amjad M, Ali S, Rizwan M (2021) Synthesis, characterization and application of novel MnO and CuO impregnated biochar composites to sequester arsenic (As) from water: modeling, thermodynamics and reusability. J Hazard Mater 401:123338
Inyang MI, Gao B, Yao Y, Xue YW, Zimmerman A, Mosa A, Pullammanappallil P, Ok YS, Cao XD (2016) A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit Rev Environ Sci Technol 46(4):406–433
Jeffery S, Verheijen FGA, van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosyst Environ 144(1):175–187
Jeon EK, Ryu S, Park SW, Wang L, Tsang DCW, Baek K (2018) Enhanced adsorption of arsenic onto alum sludge modified by calcination. J Clean Prod 176:54–62
Jimenez-Cedillo MJ, Olguin MT, Fall C, Colin-Cruz A (2013) As(III) and As(V) sorption on iron-modified non-pyrolyzed and pyrolyzed biomass from Petroselinum crispum (parsley). J Environ Manage 117:242–252
Jin HM, Capareda S, Chang ZZ, Gao J, Xu YD, Zhang JY (2014) Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: adsorption property and its improvement with KOH activation. Biores Technol 169:622–629
Johansson CL, Paul NA, de Nys R, Roberts DA (2016) Simultaneous biosorption of selenium, arsenic and molybdenum with modified algal-based biochars. J Environ Manage 165:117–123
Jovanovic BM, Vukasinovic-Pesic VL, Rajakovic LV (2011) Enhanced arsenic sorption by hydrated iron (III) oxide-coated materials-mechanism and performances. Water Environ Res 83(6):498–506
Karunanayake AG, Navarathna CM, Gunatilake SR, Crowley M, Anderson R, Mohan D, Perez F, Pittman CU, Mlsna T (2019) Fe3O4 nanoparticles dispersed on douglas fir biochar for phosphate sorption. Acs Appl Nano Mater 2(6):3467–3479
Kastner JR, Miller J, Geller DP, Locklin J, Keith LH, Johnson T (2012) Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon. Catal Today 190(1):122–132
Kim HB, Kim SH, Jeon EK, Kim DH, Tsang DCW, Alessi DS, Kwon EE, Baek K (2018) Effect of dissolved organic carbon from sludge, rice straw and spent coffee ground biochar on the mobility of arsenic in soil. Sci Total Environ 636:1241–1248
Kumar A, Bhattacharya T (2021) Removal of arsenic by wheat straw biochar from soil. Bull Environ Contam Toxicol 108:415–422
Kumarathilaka P, Seneweera S, Ok YS, Meharg AA, Bundschuh J (2020) Mitigation of arsenic accumulation in rice: an agronomical, physico-chemical, and biological approach—a critical review. Crit Rev Environ Sci Technol 50(1):31–71
Kwon JC, Nejad ZD, Jung MC (2017) Arsenic and heavy metals in paddy soil and polished rice contaminated by mining activities in Korea. CATENA 148:92–100
Lata S, Prabhakar R, Adak A, Samadder SR (2019) As(V) removal using biochar produced from an agricultural waste and prediction of removal efficiency using multiple regression analysis. Environ Sci Pollut Res 26(31):32175–32188
Lehmann J, Kuzyakov Y, Pan GX, Ok YS (2015) Biochars and the plant-soil interface. Plant Soil 395(1–2):1–5
Li SL, Wang W, Liang FP, Zhang WX (2017) Heavy metal removal using nanoscale zero-valent iron (nZVI): theory and application. J Hazard Mater 322:163–171
Li CJ, Wang JH, Yan B, Miao AJ, Zhong H, Zhang W, Ma LQ (2021a) Progresses and emerging trends of arsenic research in the past 120 years. Crit Rev Environ Sci Technol 51(13):1306–1353
Li J, Zhang Y, Wang F, Wang L, Liu J, Hashimoto Y, Hosomi M (2021b) Arsenic immobilization and removal in contaminated soil using zero-valent iron or magnetic biochar amendment followed by dry magnetic separation. Sci Total Environ 768:144521
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O’Neill B, Skjemstad JO, Thies J, Luizao FJ, Petersen J, Neves EG (2006) Black Carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70(5):1719–1730
Liang T, Li LF, Zhu CX, Liu X, Li HN, Su QQ, Ye J, Geng B, Tian YL, Sardar MF, Huang XY, Li F (2020) Adsorption of As(V) by the novel and efficient adsorbent cerium-manganese modified biochar. Water 12(10):2720
Lin LN, Qiu WW, Wang D, Huang Q, Song ZG, Chau HW (2017) Arsenic removal in aqueous solution by a novel Fe-Mn modified biochar composite: characterization and mechanism. Ecotoxicol Environ Saf 144:514–521
Lin LN, Song ZG, Huang YC, Khan ZH, Qiu WW (2019) Removal and oxidation of arsenic from aqueous solution by biochar impregnated with Fe-Mn oxides. Water Air Soil Pollut 230(5):105
Liu YY, Su GX, Zhang B, Jiang GB, Yan B (2011) Nanoparticle-based strategies for detection and remediation of environmental pollutants. Analyst 136(5):872–877
Liu J, Luo XW, Sun YQ, Tsang DCW, Qi JY, Zhang WL, Li N, Yin ML, Wang J, Lippold H, Chen YH, Sheng GD (2019a) Thallium pollution in China and removal technologies for waters: a review. Environ Int 126:771–790
Liu XW, Gao ML, Qiu WW, Khan ZH, Liu NB, Lin LN, Song ZG (2019b) Fe-Mn-Ce oxide-modified biochar composites as efficient adsorbents for removing As(III) from water: adsorption performance and mechanisms. Environ Sci Pollut Res 26(17):17373–17382
Liu J, Ren SX, Cao JL, Tsang DCW, Beiyuan JZ, Peng YT, Fang F, She JY, Yin ML, Shen NP, Wang J (2021) Highly efficient removal of thallium in wastewater by MnFe2O4-biochar composite. J Hazard Mater 401:123311
Lun MK, Lin H, He YH, Li B, Dong YB, Wang L (2019) Efficient simultaneous removal of cadmium and arsenic in aqueous solution by titanium-modified ultrasonic biochar. Biores Technol 284:333–339
Mahimairaja S, Bolan NS, Adriano DC, Robinson B (2005) Arsenic contamination and its risk management in complex environmental settings. Adv Agron 86:1–82
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154(1):29–43
Mikutta C, Mandaliev PN, Mahler N, Kotsev T, Kretzschmar R (2014) Bioaccessibility of arsenic in mining-impacted circumneutral river floodplain soils. Environ Sci Technol 48(22):13468–13477
Mo JH, Yang Q, Zhang N, Zhang WX, Zheng Y, Zhang Z (2018) A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J Environ Manage 227:395–405
Mohan D, Pittman CU (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142(1–2):1–53
Mohan D, Pittman CU, Bricka M, Smith F, Yancey B, Mohammad J, Steele PH, Alexandre-Franco MF, Gomez-Serrano V, Gong H (2007) Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interface Sci 310(1):57–73
Mohanty D (2017) Conventional as well as emerging arsenic removal technologies—a critical review. Water Air Soil Pollut 228(10):381
Mubarak NM, Sahu JN, Abdullah EC (2018) Synthesis of novel magnetic biochar using microwave heating for removal of arsenic from waste water. Iran J Chem Chem Eng Int English Edition 37(5):111–115
Mukherjee A, Zimmerman AR, Harris W (2011) Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 163(3–4):247–255
Nagodavithane CL, Singh B, Fang YY (2014) Effect of ageing on surface charge characteristics and adsorption behaviour of cadmium and arsenate in two contrasting soils amended with biochar. Soil Res 52(2):155–163
Natasha BI, Shahid M, Niazi NK, Younas F, Naqvi SR, Shaheen SM, Imran M, Wang HL, Hussaini KM, Zhang H, Rinklebe J (2021) Hydrogeochemical and health risk evaluation of arsenic in shallow and deep aquifers along the different floodplains of Punjab, Pakistan. J Hazard Mater 402:124074
Navarathna CM, Karunanayake AG, Gunatilake SR, Pittman CU, Perez F, Mohan D, Mlsna T (2019) Removal of Arsenic(III) from water using magnetite precipitated onto Douglas fir biochar. J Environ Manage 250:109429
Nguyen TH, Pham TH, Thi HTN, Nguyen TN, Nguyen MV, Dinh TT, Nguyen MP, Do TQ, Phuong T, Hoang TT, Hung TTM, Thi VH (2019) Synthesis of iron-modified biochar derived from rice straw and its application to arsenic removal. J Chem 2019:1–8
Nham NT, Al Tahtamouni TM, Nguyen TD, Huong PT, Jitae K, Viet NM, Noi NV, Phuong NM, Ahn NTH (2019) Synthesis of iron modified rice straw biochar toward arsenic from groundwater. Mater Res Exp 6(11):115528
Niazi NK, Bibi I, Shahid M, Ok YS, Burton ED, Wang HL, Shaheen SM, Rinklebe J, Luttge A (2018a) Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: an integrated spectroscopic and microscopic examination. Environ Pollut 232:31–41
Niazi NK, Bibi I, Shahid M, Ok YS, Shaheen SM, Rinklebe J, Wang HL, Murtaza B, Islam E, Nawaz MF, Luttge A (2018b) Arsenic removal by Japanese oak wood biochar in aqueous solutions and well water: Investigating arsenic fate using integrated spectroscopic and microscopic techniques. Sci Total Environ 621:1642–1651
Oremland RS, Stolz JF (2003) The ecology of arsenic. Science 300(5621):939–944
Oremland RS, Stolz JF, Hollibaugh JT (2004) The microbial arsenic cycle in Mono Lake, California. Fems Microbiol Ecol 48(1):15–27
Plewniak F, Crognale S, Rossetti S, Bertin PN (2018) A genomic outlook on bioremediation: the case of arsenic removal. Front Microbiol 9:820
Qiao JT, Liu TX, Wang XQ, Li FB, Lv YH, Cui JH, Zeng XD, Yuan YZ, Liu CP (2018) Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 195:260–271
Qiu MQ, Liu LJ, Ling Q, Cai YW, Yu SJ, Wang SQ, Fu D, Hu BW, Wang XK (2022) Biochar for the removal of contaminants from soil and water: a review. Biochar 4(1):19
Rahaman MS, Basu A, Islam MR (2008) The removal of As(III) and As(V) from aqueous solutions by waste materials. Biores Technol 99(8):2815–2823
Rahaman MS, Rahman MM, Mise N, Sikder MT, Ichihara G, Uddin MK, Kurasaki M, Ichihara S (2021) Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ Pollut 289:117940
Rajapaksha AU, Chen SS, Tsang DCW, Zhang M, Vithanage M, Mandal S, Gao B, Bolan NS, Ok YS (2016) Engineered/designer biochar for contaminant removal/immobilization from soil and water: potential and implication of biochar modification. Chemosphere 148:276–291
Regmi P, Moscoso JLG, Kumar S, Cao XY, Mao JD, Schafran G (2012) Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J Environ Manage 109:61–69
Roy P, Dey U, Chattoraj S, Mukhopadhyay D, Mondal NK (2017) Modeling of the adsorptive removal of arsenic(III) using plant biomass: a bioremedial approach. Appl Water Sci 7(3):1307–1321
Saikia R, Goswami R, Bordoloi N, Senapati KK, Pant KK, Kumar M, Kataki R (2017) Removal of arsenic and fluoride from aqueous solution by biomass based activated biochar: optimization through response surface methodology. J Environ Chem Eng 5(6):5528–5539
Sattar MS, Shakoor MB, Ali S, Rizwan M, Niazi NK, Jilani A (2019) Comparative efficiency of peanut shell and peanut shell biochar for removal of arsenic from water. Environ Sci Pollut Res 26(18):18624–18635
Scott MJ, Morgan JJ (1995) Reactions at oxide surfaces. 1. Oxidation of as(Iii) by synthetic birnessite. Environ Sci Technol 29(8):1898–1905
Serna-Chavez HM, Fierer N, van Bodegom PM (2013) Global drivers and patterns of microbial abundance in soil. Glob Ecol Biogeogr 22(10):1162–1172
Shaheen SM, Antoniadis V, Shahid M, Yang Y, Abdelrahman H, Zhang T, Hassan NEE, Bibi I, Niazi NK, Younis SA, Almazroui M, Tsang YF, Sarmah AK, Kim KH, Rinklebe J (2022a) Sustainable applications of rice feedstock in agro-environmental and construction sectors: a global perspective. Renew Sustain Energy Rev 153:111791
Shaheen SM, Mosa A, Natasha, Abdelrahman H, Niazi NK, Antoniadis V, Shahid M, Song H, Kwon EE, Rinklebe J (2022b) Removal of toxic elements from aqueous environments using nano zero-valent iron- and iron oxide-modified biochar: a review. Biochar 4(1), 24
Shaheen SM, Natasha, Mosa A, El-Naggar A, Hossain M, Abdelrahman H, Niazi NK, Shahid M, Zhang T, Tsang YF, Trakal L, Wang S, Rinklebe J (2022c) Manganese oxide-modified biochar: production, characterization and applications for the removal of pollutants from aqueous environments—a review. Bioresour Technol 346, 126581
Shaheen SM, Natasha, El-Naggar A, Hossain M, Abdelrahman H, Niazi NK, Shahid M, Zhang T, Tsang YF, Trakal L, Wang S, Rinklebe J (2022d) Managnese oxide-modified biochar: production, characterization and application for the removal of pollutants from aqueous environments—a review. Bioresour Technol 346, 126581
Shaheen SM, Niazi NK, Hassan NEE, Bibi I, Wang HL, Tsang DCW, Ok YS, Bolan N, Rinklebe J (2019) Wood-based biochar for the removal of potentially toxic elements in water and wastewater: a critical review. Int Mater Rev 64(4):216–247
Shahid M, Imran M, Khalid S, Murtaza B, Niazi NK, Zhang Y, Hussain I (2020) Arsenic environmental contamination Status in South Asia. In: Arsenic in drinking water and food. Springer Nature Singapore, pp 13–39
Shaikh WA, Alam MA, Alam MO, Chakraborty S, Owens G, Bhattacharya T, Mondal NK (2020) Enhanced aqueous phase arsenic removal by a biochar based iron nanocomposite. Environ Technol Innov 19:100936
Shaji E, Santosh M, Sarath KV, Prakash P, Deepchand V, Divya BV (2021) Arsenic contamination of groundwater: a global synopsis with focus on the Indian Peninsula. Geosci Front 12(3):101079
Shakoor MB, Bibi I, Niazi NK, Shahid M, Nawaz MF, Farooqi A, Naidu R, Rahman MM, Murtaza G, Luttge A (2018) The evaluation of arsenic contamination potential, speciation and hydrogeochemical behaviour in aquifers of Punjab, Pakistan. Chemosphere 199:737–746
Sheng GD, Li YM, Yang X, Ren XM, Yang ST, Hu J, Wang XK (2012) Efficient removal of arsenate by versatile magnetic graphene oxide composites. RSC Adv 2(32):12400–12407
Shin SY, Park SM, Baek K (2016) Electrokinetic removal of As from soil washing residue. Water Air Soil Pollut 227:223
Shrivastava A, Ghosh D, Dash A, Bose S (2015) Arsenic contamination in soil and sediment in India: sources, effects, and remediation. Curr Pollut Rep 1(1):35–46
Shrivastava A, Barla A, Singh S, Mandraha S, Bose S (2017) Arsenic contamination in agricultural soils of Bengal deltaic region of West Bengal and its higher assimilation in monsoon rice. J Hazard Mater 324:526–534
Siddiq OM, Tawabini BS, Soupios P, Ntarlagiannis D (2022) Removal of arsenic from contaminated groundwater using biochar: a technical review. Int J Environ Sci Technol 19(1):651–664
Singh R, Singh S, Parihar P, Singh VP, Prasad SM (2015) Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol Environ Saf 112:247–270
Singh P, Sarswat A, Pittman CU, Mlsna T, Mohan D (2020) Sustainable low-concentration arsenite [As(III)] removal in single and multicomponent systems using hybrid iron oxide-biochar nanocomposite adsorbents—a mechanistic study. ACS Omega 5(6):2575–2593
Srivastav AL, Pham TD, Izah SC, Singh N, Singh PK (2021) Biochar adsorbents for arsenic removal from water environment: a review. Bull Environ Contam Toxicol 108:616–628
Suliman W, Harsh JB, Abu-Lail NI, Fortuna AM, Dallmeyer I, Garcia-Perez M (2017) The role of biochar porosity and surface functionality in augmenting hydrologic properties of a sandy soil. Sci Total Environ 574:139–147
Tan XF, Liu YG, Zeng GM, Wang X, Hu XJ, Gu YL, Yang ZZ (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85
Tan XF, Liu YG, Gu YL, Xu Y, Zeng GM, Hu XJ, Liu SB, Wang X, Liu SM, Li J (2016) Biochar-based nano-composites for the decontamination of wastewater: a review. Biores Technol 212:318–333
Toth G, Hermann T, Da Silva MR, Montanarella L (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int 88:299–309
Trakal L, Michalkova Z, Beesley L, Vitkova M, Ourednicek P, Barcelo AP, Ettler V, Cihalova S, Komarek M (2018) AMOchar: amorphous manganese oxide coating of biochar improves its efficiency at removing metal(loid)s from aqueous solutions. Sci Total Environ 625:71–78
Uchimiya M, Chang S, Klasson KT (2011) Screening biochars for heavy metal retention in soil: role of oxygen functional groups. J Hazard Mater 190(1–3):432–441
Valls M, de Lorenzo V (2002) Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol Rev 26(4):327–338
Van Vinh N, Zafar M, Behera SK, Park HS (2015) Arsenic(III) removal from aqueous solution by raw and zinc-loaded pine cone biochar: equilibrium, kinetics, and thermodynamics studies. Int J Environ Sci Technol 12(4):1283–1294
Verma L, Singh J (2019) Synthesis of novel biochar from waste plant litter biomass for the removal of Arsenic (III and V) from aqueous solution: a mechanism characterization, kinetics and thermodynamics. J Environ Manage 248:109235
Villalobos M, Escobar-Quiroz IN, Salazar-Camacho C (2014) The influence of particle size and structure on the sorption and oxidation behavior of birnessite: I. Adsorption of As(V) and oxidation of As(III). Geochim Cosmochim Acta 125:564–581
Vithanage M, Herath I, Joseph S, Bundschuh J, Bolan N, Ok YS, Kirkham MB, Rinklebe J (2017) Interaction of arsenic with biochar in soil and water: a critical review. Carbon 113:219–230
Wang SS, Gao B, Li YC, Wan YS, Creamer AE (2015a) Sorption of arsenate onto magnetic iron-manganese (Fe-Mn) biochar composites. RSC Adv 5(83):67971–67978
Wang SS, Gao B, Zimmerman AR, Li YC, Ma L, Harris WG, Migliaccio KW (2015b) Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Biores Technol 175:391–395
Wang CQ, Wang H, Gu GH (2018) Ultrasound-assisted xanthation of cellulose from lignocellulosic biomass optimized by response surface methodology for Pb(II) sorption. Carbohyd Polym 182:21–28
Wei YF, Liu H, Liu CB, Luo SL, Liu YT, Yu XW, Ma JH, Yin K, Feng HP (2019a) Fast and efficient removal of As(III) from water by CuFe2O4 with peroxymonosulfate: effects of oxidation and adsorption. Water Res 150:182–190
Wei YF, Wei SD, Liu CB, Chen T, Tang YH, Ma JH, Yin K, Luo SL (2019b) Efficient removal of arsenic from groundwater using iron oxide nanoneedle array-decorated biochar fibers with high Fe utilization and fast adsorption kinetics. Water Res 167:115107
Wongrod S, Simon S, van Hullebusch ED, Lens PNL, Guibaud G (2018) Changes of sewage sludge digestate-derived biochar properties after chemical treatments and influence on As(III and V) and Cd(II) sorption. Int Biodeterior Biodegrad 135:96–102
Wongrod S, Simon S, van Hullebusch ED, Lens PNL, Guibaud G (2019) Assessing arsenic redox state evolution in solution and solid phase during As(III) sorption onto chemically-treated sewage sludge digestate biochars. Biores Technol 275:232–238
Wu C, Huang L, Xue SG, Huang YY, Hartley W, Cui MQ, Wong MH (2017) Arsenic sorption by red mud-modified biochar produced from rice straw. Environ Sci Pollut Res 24(22):18168–18178
Wu C, Cui MQ, Xue SG, Li WC, Huang L, Jiang XX, Qian ZY (2018a) Remediation of arsenic-contaminated paddy soil by iron-modified biochar. Environ Sci Pollut Res 25(21):20792–20801
Wu JZ, Huang D, Liu XM, Meng J, Tang CX, Xu JM (2018b) Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J Hazard Mater 348:10–19
Xia D, Tan F, Zhang CP, Jiang XL, Chen Z, Li H, Zheng YM, Li QB, Wang YP (2016) ZnCl2-activated biochar from biogas residue facilitates aqueous As(III) removal. Appl Surf Sci 377:361–369
Xu G, Wei LL, Sun JN, Shao HB, Chang SX (2013) What is more important for enhancing nutrient bioavailability with biochar application into a sandy soil: direct or indirect mechanism? Ecol Eng 52:119–124
Xue YW, Gao B, Yao Y, Inyang M, Zhang M, Zimmerman AR, Ro KS (2012) Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: batch and column tests. Chem Eng J 200:673–680
Yang XP, Li Q, Tang Z, Zhang WW, Yu GH, Shen QR, Zhao FJ (2017) Heavy metal concentrations and arsenic speciation in animal manure composts in China. Waste Manage 64:333–339
Yang X, Tsibart A, Nam H, Hur J, El-Naggar A, Tack FMG, Wang CH, Lee YH, Tsang DCW, Ok YS (2019) Effect of gasification biochar application on soil quality: trace metal behavior, microbial community, and soil dissolved organic matter. J Hazard Mater 365:684–694
Yang X, Hinzmann M, Pan H, Wang JX, Bolan N, Tsang DCW, Ok YS, Wang SL, Shaheen SM, Wang HL, Rinklebe J (2022a) Pig carcass-derived biochar caused contradictory effects on arsenic mobilization in a contaminated paddy soil under fluctuating controlled redox conditions. J Hazard Mater 421:126647
Yang X, Shaheen SM, Wang JX, Hou DY, Ok YS, Wang SL, Wang HL, Rinklebe J (2022b) Elucidating the redox-driven dynamic interactions between arsenic and iron-impregnated biochar in a paddy soil using geochemical and spectroscopic techniques. J Hazard Mater 422:126808
Ying SC, Kocar BD, Fendorf S (2012) Oxidation and competitive retention of arsenic between iron- and manganese oxides. Geochim Cosmochim Acta 96:294–303
Yoo JC, Beiyuan JZ, Wang L, Tsang DCW, Baek K, Bolan NS, Ok YS, Li XD (2018) A combination of ferric nitrate/EDDS-enhanced washing and sludge-derived biochar stabilization of metal-contaminated soils. Sci Total Environ 616:572–582
Yu ZH, Zhou L, Huang YF, Song ZG, Qiu WW (2015) Effects of a manganese oxide-modified biochar composite on adsorption of arsenic in red soil. J Environ Manage 163:155–162
Yu ZH, Qiu WW, Wang F, Lei M, Wang D, Song ZG (2017) Effects of manganese oxide-modified biochar composites on arsenic speciation and accumulation in an indica rice (Oryza sativa L.) cultivar. Chemosphere 168:341–349
Yu YL, Xu ZB, Xu XY, Zhao L, Qiu H, Cao XD (2021) Synergistic role of bulk carbon and iron minerals inherent in the sludge-derived biochar for As(V) immobilization. Chem Eng J 417:129183
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Biores Technol 102(3):3488–3497
Zama EF, Zhu YG, Reid BJ, Sun GX (2017) The role of biochar properties in influencing the sorption and desorption of Pb(II), Cd(II) and As(III) in aqueous solution. J Clean Prod 148:127–136
Zhang M, Gao B (2013) Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem Eng J 226:286–292
Zhang M, Gao B, Varnoosfaderani S, Hebard A, Yao Y, Inyang M (2013) Preparation and characterization of a novel magnetic biochar for arsenic removal. Biores Technol 130:457–462
Zhang QZ, Dijkstra FA, Liu XR, Wang YD, Huang J, Lu N (2014) Effects of biochar on soil microbial biomass after four Years of consecutive application in the North China Plain. PLoS ONE 9(7):102062
Zhang F, Wang X, Ji XH, Ma LJ (2016) Efficient arsenate removal by magnetite-modified water hyacinth biochar. Environ Pollut 216:575–583
Zhang W, Miao A-J, Wang N-X, Li C, Sha J, Jia J, Alessi DS, Yan B, Ok YS (2022a) Arsenic bioaccumulation and biotransformation in aquatic organisms. Environ Int 163:107221
Zhang XK, Wells M, Niazi NK, Bolan N, Shaheen S, Hou DY, Gao B, Wang HL, Rinklebe J, Wang ZY (2022b) Nanobiochar-rhizosphere interactions: Implications for the remediation of heavy-metal contaminated soils. Environ Pollut 299:118810
Zhao C, Luo KL (2018) Household consumption of coal and related sulfur, arsenic, fluorine and mercury emissions in China. Energy Policy 112:221–232
Zhong DL, Jiang Y, Zhao ZZ, Wang LL, Chen J, Ren SP, Liu ZH, Zhang YR, Tsang DCW, Crittenden JC (2019) pH Dependence of arsenic oxidation by rice-husk-derived biochar: roles of redox-active moieties. Environ Sci Technol 53(15):9034–9044
Zhong DL, Zhao ZZ, Jiang Y, Yang X, Wang LL, Chen J, Guan CY, Zhang YR, Tsang DCW, Crittenden JC (2020) Contrasting abiotic As(III) immobilization by undissolved and dissolved fractions of biochar in Ca2+-rich groundwater under anoxic conditions. Water Res 183:116106
Zhou YM, Gao B, Zimmerman AR, Cao XD (2014a) Biochar-supported zerovalent iron reclaims silver from aqueous solution to form antimicrobial nanocomposite. Chemosphere 117:801–805
Zhou YM, Gao B, Zimmerman AR, Chen H, Zhang M, Cao XD (2014b) Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Biores Technol 152:538–542
Zhou Z, Liu YG, Liu SB, Liu HY, Zeng GM, Tan XF, Yang CP, Ding Y, Yan ZL, Cai XX (2017) Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin-modified biochar. Chem Eng J 314:223–231
Zhu HJ, Jia YF, Wu X, Wang H (2009) Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J Hazard Mater 172(2–3):1591–1596
Zhu NY, Yan TM, Qiao J, Cao HL (2016) Adsorption of arsenic, phosphorus and chromium by bismuth impregnated biochar: adsorption mechanism and depleted adsorbent utilization. Chemosphere 164:32–40
Zhu NY, Zhang JH, Tang J, Zhu Y, Wu YH (2018) Arsenic removal by periphytic biofilm and its application combined with biochar. Biores Technol 248:49–55
Zimmerman AR, Gao B, Ahn MY (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43(6):1169–1179
Acknowledgements
Not applicable.
Funding
This work was supported by the Cooperative Research Program for Agriculture Science and Technology Development (PJ01475801) from Rural Development Administration, the Republic of Korea, the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2021R1A2C2011734); Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1A6A1A10045235); the National Natural Science Foundation of China (21876180); and the Outstanding Youth Project of Guangdong Natural Science Foundation (2022B1515020030). This work is also supported by a Korea University grant and OJEong Resilience Institute, Korea University.
Author information
Authors and Affiliations
Contributions
WZ: Conception, investigation, collecting and reviewing the literature, creating the tables and figures, and writing the original draft. YC: Writing, creating the tables and figures, reviewing and editing. MV: Writing, creating the tables and figures, reviewing and editing. SMS: Writing, creating the tables and figures, reviewing and editing. JR: Writing, reviewing and editing. DSA: Writing, reviewing and editing. C-H H: Writing, reviewing and editing. PAW: Writing, reviewing and editing. YSO: Conception, visualization, investigation, writing, reviewing and editing, and publication correspondence. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, W., Cho, Y., Vithanage, M. et al. Arsenic removal from water and soils using pristine and modified biochars. Biochar 4, 55 (2022). https://doi.org/10.1007/s42773-022-00181-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-022-00181-y