Abstract
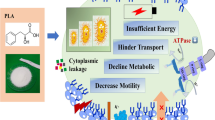
Vibrio parahaemolyticus is an important foodborne pathogenic bacterium that harbors the type III secretion system 1 (T3SS1) as an essential virulence factor. However, the pathogenesis and infection mechanism mediated by T3SS1 are not entirely clarified. Similar to previous studies on other T3SS-positive bacteria, the T3SS1 needle is a major extracellular component in V. parahaemolyticus. We recently showed that the needle gene-deletion mutant (ΔvscF) exhibited markedly decreased cytotoxicity and effector translocation during interaction with HeLa cells. To further elucidate the pathogenesis of T3SS1 during host cell infection, bacterial RNA was extracted from wild-type POR-1 and ΔvscF mutants under infected condition for comparative RNA sequencing analysis in HeLa cell. The results showed that 120 differentially expressed genes (DEGs) were identified in the ΔvscF-infected group. These encoded proteins of DEGs, such as VP2088, VP2089, and VP2091, were annotated as ABC transporter system, whereas VP0757, VP1123, and VP1289 may be new transcriptional regulators. In addition, the downregulation of T3SS1 had a positive influence on the expression of T3SS2. Moreover, the transcription of the basal body is unaffected by the needle, and there was a close relation among the tip, translocon, and needle, because bacterial adenylate cyclase two-hybrid system (BACTH system) assay indicated the interaction of VP1656, VP1670, VP1693, and VP1694 (VscF). This study provides insights into transcription mechanism of T3SS1 upon infecting HeLa cell, which is expected to better clarify the T3SS1 virulent mechanism.







Similar content being viewed by others
References
Nair GB et al (2007) Global dissemination of Vibrio parahaemolyticus serotype O3 : K6 and its serovariants. Clin Microbiol Rev 20(1):39–48
Makino K et al (2003) Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V cholerae. Lancet 361(9359):743–749
Su YC, Liu CC (2007) Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol 24(6):549–558
Broberg CA, Calder TJ, Orth K (2011) Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect 13(12–13):992–1001
Authority, E.F.S., E.F.S. Authority, and E.C.D.P. Co (2017) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. Efsa Journal 15(12):e05500
Daniels NA et al (2000) Vibrio parahaemolyticus infections in the United States, 1973–1998. J Infect Dis 181(5):1661–1666
Jung SW (2018) A foodborne outbreak of gastroenteritis caused by Vibrio parahaemolyticus associated with cross-contamination from squid in Korea. Epidemiol and Health 40:e2018056
Khimmakthong U, Sukkarun P (2017) The spread of Vibrio parahaemolyticus in tissues of the Pacific white shrimp Litopenaeus vannamei analyzed by PCR and histopathology. Microb Pathog 113:107–112
Santos MD, Orth K (2015) Subversion of the cytoskeleton by intracellular bacteria: lessons from Listeria. Salmonella and Vibrio Cellular Microbiology 17(2):164–173
Cornelis GR (2006) The type III secretion injectisome. Nat Rev Microbiol 4(11):811–825
Galan JE, Wolf-Watz H (2006) Protein delivery into eukaryotic cells by type III secretion machines. Nature 444(7119):567–573
Park KS et al (2004) Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect Immun 72(11):6659–6665
Erhardt M, Namba K, Hughes KT (2010) Bacterial nanomachines: the flagellum and type III injectisome. Cold Spring Harb Perspect Biol 2(11):a000299
Shimohata T et al (2012) VopB1 and VopD1 are essential for translocation of type III secretion system 1 effectors of Vibrio parahaemolyticus. Can J Microbiol 58(8):1002–1007
Yarbrough ML et al (2009) AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science 323(5911):269–272
Broberg CA (2011) A Vibrio effector protein is an inositol phosphatase and disrupts host cell membrane integrity (September, pg 1660, 2010). Science 331(6013):30–30
Kimbrough TG, Miller SI (2000) Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc Natl Acad Sci USA 97(20):11008–11013
Kubori T et al (2000) Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc Natl Acad Sci USA 97(18):10225–10230
Tamano K et al (2000) Supramolecular structure of the Shigella type III secretion machinery: the needle part is changeable in length and essential for delivery of effectors. EMBO J 19(15):3876–3887
Blocker A et al (2001) Structure and composition of the Shigella flexneri “needle complex”, a part of its type III secreton. Mol Microbiol 39(3):652–663
Blocker AJ et al (2008) What’s the point of the type III secretion system needle? Proc Natl Acad Sci 105(18):6507–6513
Torruellas J et al (2005) The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Mol Microbiol 57(6):1719–1733
Kato J et al (2018) A protein secreted by the Salmonella type III secretion system controls needle filament assembly. Elife 7:e35886
Cordes FS et al (2003) Helical structure of the needle of the type III secretion system of Shigella flexneri (vol 278, pg 17103, 2003). J Biol Chem 278(38):36980–36980
Hoiczyk E, Blobel G (2001) Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc Natl Acad Sci USA 98(8):4669–4674
Martinez-Argudo I, Blocker AJ (2010) The Shigella T3SS needle transmits a signal for MxiC release, which controls secretion of effectors. Mol Microbiol 78(6):1365–1378
Lian L et al (2021) VscF in T3SS1 helps to translocate VPA0226 in Vibrio parahaemolyticus. Front Cell Infect Microbiol 11(254):652432
Ohgita T, Saito H (2019) Biophysical mechanism of protein export by bacterial type III secretion system. Chem Pharm Bull 67(4):341–344
Enninga J, Rosenshine I (2009) Imaging the assembly, structure and activity of type III secretion systems. Cell Microbiol 11(10):1462–1470
Zeb S et al (2019) Type III secretion system confers enhanced virulence in clinical non-O1/non-O139 Vibrio cholerae. Microb Pathog 135:103645
Dey S et al (2019) The type III secretion system needle, tip, and translocon. Protein Sci 28(9):1582–1593
Pastor A et al (2005) PscF is a major component of the Pseudomonas aeruginosa type III secretion needle. FEMS Microbiol Lett 253(1):95–101
Ple S et al (2010) Cochaperone interactions in export of the type III needle component PscF of Pseudomonas aeruginosa. J Bacteriol 192(14):3801–3808
Plano GV, Schesser K (2013) The Yersinia pestis type III secretion system: expression, assembly and role in the evasion of host defenses. Immunol Res 57(1–3):237–245
Deng WY et al (2017) Assembly, structure, function and regulation of type III secretion systems. Nat Rev Microbiol 15(6):323–337
Park KS et al (2004) Cytotoxicity and enterotoxicity of the thermostable direct hemolysin-deletion mutants of Vibrio parahaemolyticus. Microbiol Immunol 48(4):313–318
Karimova G et al (1998) A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci USA 95(10):5752–5756
Battesti A, Bouveret E (2012) The bacterial two-hybrid system based on adenylate cyclase reconstitution in Escherichia coli. Methods 58(4):325–334
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Chong L (2001) Molecular cloning - a laboratory manual, 3rd edition. Science 292(5516):446–446
Karimova G, Ullmann A, Ladant D (2000) Bacterial two-hybrid system that exploits a cAMP signaling cascade in Escherichia coli. Applications of Chimeric Genes and Hybrid Proteins, Pt C 328:59–73
Karimova G, Dautin N, Ladant D (2005) Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J Bacteriol 187(7):2233–2243
Kusmierek M et al (2019) A bacterial secreted translocator hijacks riboregulators to control type III secretion in response to host cell contact. Plos Pathogens 15(6):e1007813
Zhu CH et al (2017) Genomic and transcriptomic analyses reveal distinct biological functions for cold shock proteins (VpaCspA and VpaCspD) in Vibrio parahaemolyticus CHN25 during low-temperature survival. Bmc Genomics 18(1):436
Cornforth DM et al (2018) Pseudomonas aeruginosa transcriptome during human infection. Proc Natl Acad Sci USA 115(22):E5125–E5134
Wang Q, Chen MM, Zhang W (2018) A two-component signal transduction system contributes to the virulence of Riemerella anatipestifer. J Vet Sci 19(2):260–270
Nydam SD, Shah DH, Call DR (2014) Transcriptome analysis of Vibrio parahaemolyticus in type III secretion system 1 inducing conditions. Front Cell Infect Microbiol 4:1
Srisanga K et al (2019) Polyphosphate kinase 1 of Burkholderia pseudomallei controls quorum sensing, RpoS and host cell invasion. J Proteomics 194:14–24
Bohn E et al (2019) Bacterial adhesion and host cell factors leading to effector protein injection by type III secretion system. Int J Med Microbiol 309(5):344–350
Abisado RG et al (2018) Bacterial quorum sensing and microbial community interactions. Mbio 9(5):e02331-17
Whiteley M, Diggle SP, Greenberg EP (2017) Progress in and promise of bacterial quorum sensing research. Nature 551(7680):313–320
Matilla MA, Krell T (2018) The effect of bacterial chemotaxis on host infection and pathogenicity. FEMS Microbiol Rev 42(1):40–67
de Carvalho CCCR, Caramujo MJ (2018) The various roles of fatty acids. Molecules 23(10):2583
Lewis VG, Ween MP, McDevitt CA (2012) The role of ATP-binding cassette transporters in bacterial pathogenicity. Protoplasma 249(4):919–942
Wang CY et al (2018) ABC transporter content diversity in Streptococcus pneumoniae impacts competence regulation and bacteriocin production. Proc Natl Acad Sci USA 115(25):E5776–E5785
Osborne SE et al (2012) Characterization of DalS, an ATP-binding cassette transporter for D-alanine, and its role in pathogenesis in Salmonella enterica. J Biol Chem 287(19):15242–15250
Park KS et al (2000) Genetic characterization of DNA region containing the trh and ure genes of Vibrio parahaemolyticus. Infect Immun 68(10):5742–5748
Hiles ID et al (1987) Molecular characterization of the oligopeptide permease of salmonella-Typhimurium. J Mol Biol 195(1):125–142
Masukagami Y et al (2018) Metabolite profiling of Mycoplasma gallisepticum mutants, combined with bioinformatic analysis, can reveal the likely functions of virulence-associated genes. Vet Microbiol 223:160–167
Nguyen PT et al (2018) Noncanonical role for the binding protein in substrate uptake by the MetNI methionine ATP binding cassette (ABC) transporter. Proc Natl Acad Sci USA 115(45):E10596–E10604
Xu M, Wang J, Mou HJ (2015) Fatty acid profiles of Vibrio parahaemolyticus and its changes with environment. J Basic Microbiol 55(1):112–120
Masoudi A et al (2014) Chasing acyl carrier protein through a catalytic cycle of lipid A production. Nature 505(7483):422–6
Zhang YQ et al (2019) QsvR integrates into quorum sensing circuit to control Vibrio parahaemolyticus virulence. Environ Microbiol 21(3):1054–1067
Teh PP et al (2016) The transcriptional regulator, IRF4 is essential for the development of follicular T regulatory cells. Eur J Immunol 46:745–745
Toren E et al (2020) The Ldb1 transcriptional co-regulator is required for establishment, development, and survival of the pancreatic endocrine lineage. Faseb Journal 34(s1):1
Sun FJ et al (2014) H-NS is a repressor of major virulence gene loci in Vibrio parahaemolyticus. Front Microbiol 5:675
Gao H et al (2017) Transcriptional regulation of cpsQ-mfpABC and mfpABC by CalR in Vibrio parahaemolyticus. Microbiologyopen 6(4):e00470
Kodama T et al (2015) Regulation of Vibrio parahaemolyticus T3SS2 gene expression and function of T3SS2 effectors that modulate actin cytoskeleton. Cell Microbiol 17(2):183–190
Zhang YQ et al (2016) Transcription of exsD is repressed directly by H-NS in Vibrio parahaemolyticus. Microb Pathog 97:221–225
Kodama T et al (2010) Two regulators of vibrio parahaemolyticus play important roles in enterotoxicity by controlling the expression of genes in the Vp-PAI region. Plos One 5(1):e8678
Osei-Adjei G et al (2017) Regulatory actions of ToxR and CalR on their own genes and type III secretion system 1 in Vibrio parahaemolyticus. Oncotarget 8(39):65809–65822
Zhang YQ et al (2018) Autoregulation of ToxR and its regulatory actions on major virulence gene loci in Vibrio parahaemolyticus. Front Cell Infect Microbiol 8:291
Whitaker WB et al (2012) The Vibrio parahaemolyticus ToxRS regulator is required for stress tolerance and colonization in a novel orogastric streptomycin-induced adult murine model. Infect Immun 80(5):1834–1845
Ibarra JA, Steele-Mortimer O (2009) Salmonella- the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cellular Microbiology 11(11):1579–1586
LaRock DL, Chaudhary A, Miller SI (2015) Salmonellae interactions with host processes. Nat Rev Microbiol 13(4):191–205
Figueira R, Holden DW (2012) Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology-Sgm 158:1147–1161
Gode-Potratz CJ, Chodur DM, McCarter LL (2010) Calcium and iron regulate swarming and type III secretion in Vibrio parahaemolyticus. J Bacteriol 192(22):6025–6038
Gotoh K et al (2010) Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants. Plos One 5(10):e13365
Li P et al (2016) Bile salt receptor complex activates a pathogenic type III secretion system. Elife 5:e15718
Yin LX et al (2020) Characterization of a membrane binding loop leads to engineering botulinum neurotoxin B with improved therapeutic efficacy. Plos Biology 18(3):e3000618
Tao L et al (2017) Engineered botulinum neurotoxin B with improved efficacy for targeting human receptors. Nature Communications 8:53
Montagner C, Arquint C, Cornelis GR (2011) Translocators YopB and YopD from Yersinia enterocolitica form a multimeric integral membrane complex in eukaryotic cell membranes. J Bacteriol 193(24):6923–6928
Mattei PJ et al (2011) Membrane targeting and pore formation by the type III secretion system translocon. FEBS J 278(3):414–426
Wagner S et al (2010) Length control of the injectisome needle requires only one molecule of Yop secretion protein P (YscP). Proc Natl Acad Sci USA 107(31):13860–13865
Agrain C et al (2005) Secretion of YscP from Yersinia enterocolitica is essential to control the length of the injectisome needle but not to change the type III secretion substrate specificity. Mol Microbiol 57(5):1415–1427
Souza CD et al (2018) The YscE/YscG chaperone and YscF N-terminal sequences target YscF to the Yersinia pestis type III secretion apparatus. Microbiology-Sgm 164(3):338–348
Sun P et al (2008) Structural characterization of the Yersinia pestis type III secretion system needle protein YscF in complex with its heterodimeric chaperone YscE/YscG. Faseb Journal 377(3):819–30
Ho O et al (2017) Characterization of the ruler protein interaction interface on the substrate specificity switch protein in the Yersinia type III secretion system. J Biol Chem 292(8):3299–3311
Deane JE et al (2006) Molecular model of a type III secretion system needle: implications for host-cell sensing. Proc Natl Acad Sci USA 103(33):12529–12533
Acknowledgements
We thank ENAGO (www.enago.cn) for providing linguistic assistance during the preparation of this manuscript and Dr. Dejun Ji (Yangzhou University) for useful comments on checking spelling and grammar. We also thank DECODE GENOMICE Biotechnology Corporation for Transcriptome sequencing.
Funding
This study was funded by the National Natural Science Foundation of China (31871893), the National Key Research and Development Program of China (2017YFF0208600), the Jiangsu Agricultural Independent Innovation Project (SCX (18) 2011), the National “Youth Top-notch Talent” Support Program (W0270187), the Introduction of Nanjing Agricultural University Scientific Research Grants Project (804121), the Central Guidance for Local Science and Technology Development (No. YDZX20173100004528), the Science and Technology Joint Project of the Yangtze River Delta (No.17395810102), the Jiangsu Collaborative Innovation Center of Meat Production and Processing, and the Yangzhou Hanjiang Science and Technology Bureau program (2020).
Author information
Authors and Affiliations
Contributions
Lele Lian: conceptualization; data curation; writing original draft. Wanjun Li, Tingyue Xue: formal analysis. Jianluan Ren, Fang Tang, Yongjie Liu: project administration. Feng Xue, Jianjun Dai: conceptualization; funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
This article does not present any study involving human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lian, L., Li, W., Xue, T. et al. Comparative transcriptomic analysis provides insights into transcription mechanisms of Vibrio parahaemolyticus T3SS during interaction with HeLa cells. Braz J Microbiol 53, 289–301 (2022). https://doi.org/10.1007/s42770-021-00627-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00627-8