Abstract
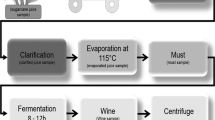
Brazil is the second largest ethanol producer in the world and largest using sugarcane feedstock. Bacteria contamination is one of the most important issues faced by ethanol producers that seek to increase production efficiency. Each step of production is a selection event due to the environmental and biological changes that occur. Therefore, we evaluated the influence of the selection arising from the ethanol production process on diversity and composition of bacteria. Our objectives were to test two hypotheses, (1) that species richness will decrease during the production process and (2) that lactic acid bacteria will become dominant with the advance of ethanol production. Bacterial community assemblage was accessed using 16S rRNA gene sequencing from 19 sequential samples. Temperature is of great importance in shaping microbial communities. Species richness increased between the decanter and must steps of the process. Low Simpson index values were recorded at the fermentation step, indicating a high dominance of Lactobacillus. Interactions between Lactobacillus and yeast may be impairing the efficiency of industrial ethanol production.





Similar content being viewed by others
References
Lopes ML, Paulillo SC d L, Godoy A et al (2016) Ethanol production in Brazil: a bridge between science and industry. Braz J Microbiol 47:64–76. https://doi.org/10.1016/j.bjm.2016.10.003
Amorim HV, Lopes ML, De Castro Oliveira JV et al (2011) Scientific challenges of bioethanol production in Brazil. Appl Microbiol Biotechnol 91:1267–1275. https://doi.org/10.1007/s00253-011-3437-6
Basso L, Basso T, Rocha S (2011) Ethanol production in Brazil: the industrial process and its impact on yeast fermentation. Biofuel Prod - Recent Dev Prospect 1530:85–100. https://doi.org/10.5772/959
Costa OYA, Souto BM, Tupinambá DD et al (2015) Microbial diversity in sugarcane ethanol production in a Brazilian distillery using a culture-independent method. J Ind Microbiol Biotechnol 42:73–84. https://doi.org/10.1007/s10295-014-1533-1
Bischoff KM, Liu S, Leathers TD et al (2009) Modeling bacterial contamination of fuel ethanol fermentation. Biotechnol Bioeng 103:117–122. https://doi.org/10.1002/bit.22244
Brexó RP, Sant’ Ana A d S (2018) Microbial interactions during sugar cane must fermentation for bioethanol production: does quorum sensing play a role? Crit Rev Biotechnol 38:231–244. https://doi.org/10.1080/07388551.2017.1332570
Worley-Morse TO, Deshusses MA, Gunsch CK (2015) Reduction of invasive bacteria in ethanol fermentations using bacteriophages. Biotechnol Bioeng 112:1544–1553. https://doi.org/10.1002/bit.25586
Muthaiyan A, Limayem A, Ricke SC (2011) Antimicrobial strategies for limiting bacterial contaminants in fuel bioethanol fermentations. Prog Energy Combust Sci 37:351–370. https://doi.org/10.1016/J.PECS.2010.06.005
Bayrock DP, Ingledew WM (2004) Inhibition of yeast by lactic acid bacteria in continuous culture: nutrient depletion and/or acid toxicity? J Ind Microbiol Biotechnol 31:362–368. https://doi.org/10.1007/s10295-004-0156-3
Castro REN de, Alves RM de B, Nascimento CAO do, Giudici R (2019) Assessment of Sugarcane-Based Ethanol Production. In: Basso TP, Basso LC (ed) Fuel Ethanol Production from Sugarcane. 1st edn. IntechOpen, pp 3–21. https://doi.org/10.5772/intechopen.78301
Lucena BTL, Dos Santos BM, Moreira JLS et al (2010) Diversity of lactic acid bacteria of the bioethanol process. BMC Microbiol 10. https://doi.org/10.1186/1471-2180-10-298
Bonatelli ML, Quecine MC, Silva MS, Labate CA (2017) Characterization of the contaminant bacterial communities in sugarcane first-generation industrial ethanol production. FEMS Microbiol Lett 364. https://doi.org/10.1093/femsle/fnx159
Vellend M (2010) Conceptual synthesis in community ecology. Q Rev Biol 85:183–206. https://doi.org/10.1086/652373
Hanson CA, Fuhrman JA, Horner-Devine MC, Martiny JBH (2012) Beyond biogeographic patterns: processes shaping the microbial landscape. Nat Rev Microbiol 10:497–506. https://doi.org/10.1038/nrmicro2795
Lenski RE (2017) Experimental evolution and the dynamics of adaptation and genome evolution in microbial populations. ISME J:1–14. https://doi.org/10.1038/ismej.2017.69
Klindworth A, Pruesse E, Schweer T et al (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41:e1. https://doi.org/10.1093/nar/gks808
Pylro VS, Roesch LFW, Morais DK et al (2014) Data analysis for 16S microbial profiling from different benchtop sequencing platforms. J Microbiol Methods 107:30–37. https://doi.org/10.1016/j.mimet.2014.08.018
Rognes T, Flouri T, Nichols B et al (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. https://doi.org/10.7717/peerj.2584
Caporaso JG, Kuczynski J, Stombaugh J et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Quast C, Pruesse E, Yilmaz P et al (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:590–596. https://doi.org/10.1093/nar/gks1219
Fay MP, Proschan MA (2010) Wilcoxon-Mann-Whitney or t-test? On assumptions for hypothesis tests and multiple interpretations of decision rules. Stat Surv 4:1–39. https://doi.org/10.1214/09-SS051
Murtagh F, Legendre P (2014) Ward ’ s hierarchical agglomerative clustering method : which algorithms implement ward ’ s criterion ? J Classif 31:274–295. https://doi.org/10.1007/s00357
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.Rproject.org/
Wickham H (2016) ggplot2. Springer International Publishing, Cham
McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. https://doi.org/10.1371/journal.pone.0061217
Oksanen J, Blanchet FG, Friendly M, et al (2019) vegan: Community Ecology Package. R package version 2.5–5. https://CRAN.Rproject.org/package=vegan
Skinner KA, Leathers TD (2004) Bacterial contaminants of fuel ethanol production. J Ind Microbiol Biotechnol 31:401–408. https://doi.org/10.1007/s10295-004-0159-0
Hill M (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432. https://doi.org/10.2307/1934352
Haegeman B, Hamelin J, Moriarty J et al (2013) Robust estimation of microbial diversity in theory and in practice. ISME J 7:1092–1101. https://doi.org/10.1038/ismej.2013.10
Kamboj K, Vasquez A, Balada-Llasat JM (2015) Identification and significance of Weissella species infections. Front Microbiol 6:1–7. https://doi.org/10.3389/fmicb.2015.01204
Fairfax MR, Lephart PR, Salimnia H (2014) Weissella confusa: problems with identification of an opportunistic pathogen that has been found in fermented foods and proposed as a probiotic. Front Microbiol 5:1–5. https://doi.org/10.3389/fmicb.2014.00254
Fusco V, Quero GM, Cho GS et al (2015) The genus Weissella: taxonomy, ecology and biotechnological potential. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.00155
Hammes WP, Vogel RF (1995) The genus Lactobacillus. In: Wood BJB, Holzapfel WH (eds) The genera of lactic acid bacteria. Springer US, Boston, pp 19–54
Goh KM, Gan HM, Chan KG et al (2014) Analysis of Anoxybacillus genomes from the aspects of lifestyle adaptations, prophage diversity, and carbohydrate metabolism. PLoS One 9. https://doi.org/10.1371/journal.pone.0090549
Studholme DJ (2015) Some (bacilli) like it hot: genomics of Geobacillus species. Microb Biotechnol 8:40–48. https://doi.org/10.1111/1751-7915.12161
Zeigler DR (2014) The Geobacillus paradox: why is a thermophilic bacterial genus so prevalent on a mesophilic planet? Microbiology (United Kingdom) 160:1–11. https://doi.org/10.1099/mic.0.071696-0
Goh KM, Kahar UM, Chai YY et al (2013) Recent discoveries and applications of Anoxybacillus. Appl Microbiol Biotechnol 97:1475–1488. https://doi.org/10.1007/s00253-012-4663-2
Doughari HJ, Ndakidemi PA, Human IS, Benade S (2011) The ecology, biology and pathogenesis of Acinetobacter spp.: an overview. Microbes Environ 26:101–112. https://doi.org/10.1264/jsme2.ME10179
Rogers PL, Lee KJ, Skotnicki ML, Tribe DE (1982) Ethanol production by Zymomonas mobilis. In: Microbial Reactions. Advances in Biochemical Engineering. Springer, Berlin, Heidelberg, pp 37–84
Swings J, De Ley J (1977) The biology of Zymomonas. Bacteriol Rev 41:1–46
Aris JP, Benner SA, Thomson JM et al (2005) Resurrecting ancestral alcohol dehydrogenases from yeast. Nat Genet 37:630–635. https://doi.org/10.1038/ng1553
Basso TO, Oliveira Lino FS de (2019) Clash of Kingdoms: How Do Bacterial Contaminants Thrive in and Interact with Yeasts during Ethanol Production? In: Fuel Ethanol Production from Sugarcane. In: Basso TP, Basso LC (ed) Fuel Ethanol Production from Sugarcane. 1st edn. IntechOpen, pp 3–21. https://doi.org/10.5772/intechopen.78413
Narendranath NV, Power R (2005) Relationship between pH and medium dissolved solids in terms of growth and metabolism of lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl Environ Microbiol 71:2239–2243. https://doi.org/10.1128/AEM.71.5.2239-2243.2005
Narendranath NV, Hynes SH, Thomas KC, Ingledew WM (1997) Effects of lactobacilli on yeast-catalyzed ethanol fermentations. Appl Environ Microbiol 63:4158–4163
Acknowledgments
We would like to thank Bruno Spacek for critical reviews of the manuscript.
Funding
This work was granted by Instituto Federal de Minas Gerais, Edital de Pesquisa Aplicada no. 156/2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eleni Gomes.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1012 kb)
Rights and permissions
About this article
Cite this article
Queiroz, L.L., Costa, M.S., de Abreu Pereira, A. et al. Dynamics of microbial contaminants is driven by selection during ethanol production. Braz J Microbiol 51, 303–312 (2020). https://doi.org/10.1007/s42770-019-00147-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-019-00147-6