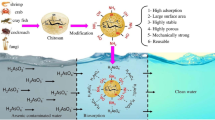
Abstract
Arsenic (As) contamination in drinking water is a global concern. Development of facile and low cost As remediation system is of significant social and economic interest. Here, we report a cost-effective and water-stable transition metal oxide doped cross-linked chitosan cryogel for highly sensitive removal of As ion from potable water. Sample aliquots were tested for total arsenic by inductively coupled plasma mass spectroscopy. It was found that the sorption kinetics follows Freundlich isotherm model. We observe remarkably high arsenic removal efficiency (76%) with only 2 h of contact time with cryogel. The scaffold materials can be easily regenerated with acetone. The high removal efficiency of As metal and recyclability of this novel synthesized metal oxide doped cross linked chitosan cryogel render them a potential candidate for low cost arsenic removal based filter development.
Similar content being viewed by others
1 Introduction
Arsenic (As) pollution in water is a global concern due to their adverse impact on the environment and human health [1, 2]. According to World Health Organisation (WHO), the maximum allowed contaminant level for total arsenic in potable water is determined to be 10 ppb [3].
Various treatment methods such as chemical precipitation, membrane filtration [4], oxidation-precipitation [5], coagulation-flocculation associated to filtration [6], sorption [7, 8], nanofiltration [9, 10], ion exchange [11], reverse osmosis [12], etc. have been utilized for the removal of As from contaminated potable water. Among all these methods, adsorption is considered as one of the most suitable water remediation methods because it is more effective and cost effective and can be applied for the removal of different types of pollutants [13,14,15].
During the last few years, chitosan as a natural biopolymer has extensively used in water remediation and pharmaceutical industry as a scaffold material and it satisfies certain functional properties of an effective adsorbent [16, 17]. It is obtained by alkaline deacetylation of chitin and used as an adsorbent material because of its biodegradability, bioactivity, biocompatibility, hydrophilicity, nontoxicity, low cost and abundance in nature and most importantly due to the presence of a large number of functional groups (–OH, –NH2) which act as metal binding sites. But pristine chitosan has many disadvantages like instability in acidic medium, low thermal stability, low mechanical strength, inadequate porosity and surface area which restricts its potential use as an adsorbent in water filtration industry. This can be circumvented by various physical and chemical modifications in the pristine chitosan which can increase its efficiency in heavy metal ion removal [18]. For instance, graphene-based chitosan xerogel acts as a preferred material for heavy metals like Pb(II), Cd(II), Hg(II) removal [19]. A highly plausible method of treatment of As in waste water having acidic condition is also investigated using a UV light driven sulfuration agent [20]. In another study, the removal of arsenate from aqueous system using iron-chitosan coated sand shows a high efficacy at near neutral pH. Apart from the available functionalities of chitosan, the immobilized coating of iron supplements the arsenate adsorption by formation of hydrate iron oxide on sand surface [21]. The adsorption capacity depends on various physiochemical properties such as availability of accessible amine groups, polymer weight, and crystallinity. The amine group present in chitosan reacts strongly with metal cations through chelation mechanism at neutral pH. In acidic condition, amine groups uptake metal anions by electrostatic attraction [22]. To increase stability under experimental conditions, chitosan is subjected to chemical cross-link with different polymers. Among various cross linkers, succinic acid acts as a biocompatible crosslinker/stabilizer to the chitosan matrix which increases its mechanical stability many folds. It interacts with chitosan non-covalently i.e. both ionically and through intermolecular hydrogen bonds [23]. By modifying chitosan physically [24] and chemically [22], its sorption capacity, mechanical and chemical stability can be enhanced. Although crosslinking increases its mechanical stability, it is also important to enhance its porosity for successful removal of heavy metal ions. Previously, it is reported that metal oxide doping can significantly increase polymer matrix porosity. For example, Cu(OH)2 and CuO offers sorption sites which has a strong affinity for arsenate. Under controlled pH environment, CuO and encapsulating chitosan matrix adsorb As through anion exchange/electrostatic attraction [1]. When combined with polymers, CuO forms composites with unique chemical and physical properties. Similarly, CuO–ZnO nanofibers have a very high affinity for arsenate anions than only ZnO nanofibres. Other anions in natural water do not interfere much in adsorption process which proves the significance of CuO–ZnO in As removal [25]. Apart from this, ZnO has its uses as a good antibacterial agent. It is resilient to harsh processing conditions, durable, heat resistant and selective. These properties pave the way for its potential use as a water disinfectant [26].
Here, we report the synthesis and As removal application of CuO–ZnO doped cross-linked chitosan. Chitosan itself is a good sorbent for cationic metal removal but its adsorption capacity for As is significantly less that puts a constraint on its use by itself [27]. However, CuO–ZnO doping as reported here enhances the adsorption capacity to many folds. We choose CuO because pH adjustments of solution are not required in the case of arsenic removal. It also helps in oxidation of arsenite(III) to arsenate(V). At neutral pH As(III) contains uncharged species (H3AsO3) which is unable to react with functionalities present in adsorbents whereas As(V) contains monovalent (H2AsO4−) and divalent (HAsO42−) form [28]. Moreover, CuO shows antibacterial properties in some particular cases and it has an extremely high surface area as well [29]. In addition to this ZnO is considered to be a very stable, non-toxic and user-friendly semiconductor oxide with antibacterial property.
The synthesized cryogel have been characterized by Fourier transformation infrared spectroscopy (FTIR), Thermo gravimetric analysis (TGA), Field emission scanning electron microscopy (FESEM), Energy dispersive X-ray analysis (EDX), Powder X-ray diffraction (PXRD) etc. and employed as a stable adsorption matrix for the As removal. The concentration of As present in the water before and after adsorption process has been measured by Inductively coupled plasma mass spectrophotometer (ICP-MS) studies. The residual effluents concentrations as a function of contact time are measured. As removal efficiency as high as 76% with only 2 h of contact time with cryogel was observed. The mechanism of arsenic removal was established by SEM, XRD and FTIR studies. More interestingly, our designed cryogel can be regenerated using acetone treatment.
2 Materials and methods
2.1 Chemicals
Chitosan powder (75–85% deacetylated) and succinic acid were procured from Sigma Aldrich (USA). Laboratory grade Zinc oxide (pure) and Cupric(II) oxide powder were supplied by S D Fine-Chem limited. Analytical grade Sodium arsenate was obtained from Thomas Baker. It was used for preparing arsenic solutions. Deionized water was used throughout the experiments and all the chemicals were used without further purification.
2.2 Synthesis
The chitosan-succinic acid hydrogel was prepared by a previously reported freeze-drying process with some modifications [23]. Chitosan powder (1%) was added to 20 ml of deionized water and stirred in a magnetic stirrer. Then succinic acid (0.5% w/v) was slowly added to the previous solution and stirred vigorously for 3–4 h at room temperature. After completion of the reaction, the solution was subjected to centrifugation at 7000 rpm for 20 min. The solution was frozen at 0 °C for 2 h first and then at − 80 °C for 12 h. Following freezing, the sample was subjected to lyophilization for 7 h at − 80 °C using a lyophilizer. Then the cryogel was washed with 0.05 N NaOH and ethanol mixture and then sonicated for 5 min. This step was followed for three times and the material was further washed with water and ethanol (7:3 ratio) mixture and subjected to lyophilize at − 80 °C.
For the preparation of CuO–ZnO doped cryogel, 1:1 ratio of CuO and ZnO was added to the chitosan solution, stirred for 2 h for stable dispersion followed by addition of succinic acid. Then the as-reported procedure for the chitosan gel was followed to prepare the scaffold. The material could be regenerated using acetone and it could be used up to 2 cycles. We abbreviate the chitosan-succinic acid scaffold material as CH-SA, CuO–ZnO doped chitosan-succinic acid scaffold material as CH-SA-CZ in this manuscript. The arsenic adsorbed CH-SA-CZ is abbreviated as CH-SA-CZ-As. The schematic representation of the synthesis procedure is shown in Scheme 1.
3 Characterization
The PXRD pattern of CH-SA, CH-SA-CZ, and arsenic adsorbed CH-SA-CZ was recorded using a Rigaku powder X-ray diffractometer. The PXRD data were scanned with a 2θ range of 5°–90°. The morphology of the samples was studied using scanning electron micrograph LEO 435 VP tungsten filament-based SEM instrument under high voltage of 15 kV with different magnifications. FTIR spectroscopy was carried out to analyze the functional groups present in the samples. All spectra were recorded in the wave number range of 450–4000 cm−1. TGA was carried out with SII 6300 EXSTAR instrument and EDX was taken with FE-SEM QUANTA 200 FEG instrument. Quantification of As(V) concentration prior and later to the adsorption experiment was done using Perkin Elmer ELAN DRC-e ICP-MS instrument. The instrument was calibrated using 3 standard solutions of 0.1 ppm, 0.5 ppm, and 1 ppm.
4 Arsenic removal study
Arsenic stock solution was prepared by dissolving 10 mg of sodium arsenate in 10 ml of deionized water. Now arsenic solutions of various concentrations were prepared by diluting the stock solution with deionized water. Adsorption experiments were performed using 5 ml of 1 mg/l (1 ppm) arsenic solution as pollutant solution. 10 mg of sorbent materials were added to the pollutant solution through the following procedure.
5 ml of 1 ppm Arsenic solution were taken in 8 vials. 10 mg each of CH-SA were put in 4 vials and 10 mg each of CH-SA-CZ were put in another 4 vials. The sorbents were taken out of the solutions at a regular time interval of 15 min, 30 min, 1 h and 2 h and the residual concentration of pollutants were monitored using ICP-MS.
For regeneration, the sorbent that was taken out after 30 min was dipped in 10 ml acetone and then washed with deionized water. The washed material was put in oven at 100 °C for 15 min. Then the regenerated material was employed for As removal by dipping into 5 ml of fresh 1 ppm arsenic solution for 30 min. The same procedure was repeated for another cycle.
4.1 Adsorption study
To investigate the arsenic removal capacity of CH-SA-CZ, adsorption studies were performed through a batch experiment. 20 mg of adsorbent CH-SA-CZ was dipped in 40 ml of arsenic solution with initial concentration 1.7 ppm. 5 ml aliquots were taken out at predetermined time intervals (t) for kinetic study and were analyzed using ICPMS (Inductively coupled plasma mass spectroscopy). The adsorbed arsenic concentration after a particular time (qt in mg/g) was calculated from the following equation:
where Co is initial concentration of arsenic in mg/l and Ct is concentration of arsenic after time t. V represents the volume and m (mg) denotes the mass of the adsorbent used.
To study the adsorption isotherm, arsenic solutions of different concentrations were prepared by diluting the 1000 ppm stock arsenic solution with deionized water. 5 mg of CH-SA-CZ adsorbent were added to 10 ml of each of the solutions and stirred for 24 h. The solutions were analyzed by ICPMS to detect the final arsenic concentration. The following equation was used to find out the adsorption capacity (qe in mg/g) of CH-SA-CZ adsorbent:
5 Results and discussion
The morphologies of the CH-SA, and CH-SA-CZ from SEM images are shown in Fig. 1. Here, morphological features of the CH-SA (Fig. 1a), and CH-SA-CZ (Fig. 1b) like pore sizes and their average distribution are compared. EDX measurements support the incorporation of CuO and ZnO particles in the studied cryogel structures (Figure S1). It is observed that the average pore size and number of pores of the prepared CH-SA-CZ material is much higher than CH-SA. More than 40% of the matrix area of CH-SA-CZ is covered with pores which are higher than that for standard CH-SA material. The same trend follows in the case of CuO and ZnO doped cross-linked chitosan namely CH-SA-Cu and CH-SA-Zn respectively. In both these cases, we observe increase in pore size and the higher distribution of pores than standard chitosan (supplementary information, Table S1). This highly porous, well-distributed and inter-connected scaffold material structure can serve as a spongy matrix for adsorption of metal ions. In order to have better understanding of morphology of the adsorbent, we have incorporated TEM images of CH-SA-CZ cryogel along with CuO and ZnO powders (Figure S2).
Figure 2a shows XRD patterns of CH-SA and CH-SA-CZ. Chitosan powder exhibits main diffraction peaks at 2θ = 11.6° and 20.25°. The cross-linked CH-SA-CZ depicts diffraction peaks at 2θ = 11.9° and 22.3°. Incorporation of metal oxides decreases the crystallinity of the as fabricated cryogel and the peak is shifted to higher 2θ (22.3°) value. Also diffraction peaks corresponding to metal oxides are introduced in the cryogel (Figure S3). We have obtained peak corresponding to CuO at 2θ = 39.3° that is ascribed to [111] plane. The diffraction peak at 2θ = 36.08° in CH-SA-CZ corresponds to [101] plane of ZnO. These results also suggest that CH-SA-CZ has good compatibility, which leads to the formation of a porous type network which may participate in metal ion removal [30].
Furthermore, CuO and ZnO doping does not change the functional group availability of succinic acid cross-linked chitosan cryogel necessary for efficient metal ion adsorption as shown in FTIR spectrum (Fig. 2b). We do not observe any significant peak shift in CH-SA and CH-SA-CZ vibrational spectrum. The predominant peak at 3134 cm−1 indicates –OH and –NH stretching vibrations whether the peak at 1630 cm−1 and 1400 cm−1 depicts the –C = O in amide group and –C–O–H in-plane bending respetively. –NH bending in vibration mode is represented by the 1560 cm−1 peak. The peak at 1192 cm−1 and 1110 cm−1 appear due to –C–O–C bond and –C–O stretch [23].
The TGA experiments of CH-SA and CH-SA-CZ cryogel are also carried out in order to confirm their thermal stability (Fig. 3). Both the studied compounds have been found to be degraded by approximately 10% around 100 °C. This can be mainly attributed to the removal of moisture trapped inside chitosan matrix. Up to ~ 200 °C, both the composite materials have been found to be relatively stable. Beyond the temperature, composite materials have been degraded quite drastically. This could be due to the degradation of functionalities along with the basic backbone structure. The results indicate that incorporation of CuO and ZnO did not change the primary structure of the composition and have less contribution to the thermal stability of the studied materials (Table S2). This highly mechanically and thermally stable chitosan cryogel renders themselves as a suitable candidate for efficient water remediation process even at relatively higher temperature. The exothermic peak after 400 °C corresponds to the thermal decomposition of pyranose ring and degradation of residual carbon in chitosan. Shifting of the peak towards lower temperature in CH-SA-CZ may be attributed to the chelation of chitosan with metal ions [31].
6 Arsenic removal study
6.1 ICPMS studies
Arsenic adsorption studies are carried out by dipping chitosan-based cryogel CH-SA and CH-SA-CZ into the standard Arsenic solution respectively and the residual concentrations of As are measured at regular interval of time by ICPMS. The concentration of effluents as a function of time is measured in both cases. Here, chitosan-based cryogels are dipped into the pollutant (As) solutions for certain periods of time and after removing the concentration of the remaining effluents are measured. The ICPMS study shows that only CH-SA scaffold did not adsorb arsenic significantly as the arsenic concentration was lowered down from 1.17 ppm to only 1.16 ppm after 15 min contact time and eventually to 1.04 ppm after 2 h. On a further note, it is also observed that the subjected CH-SA matrix is not stable beyond 2 h. On the other hand, we observe a significant enhancement in the adsorption capacity of metal oxide doped cryogel CH-SA-CZ. The residual pollutant concentrations decrease from 1.17 ppm to 0.89 ppm only after 15 min of contact time and a remarkably lower value of 0.27 ppm after 2 h of contact time (Fig. 4). Interestingly, the CH-SA-CZ matrix is also stable beyond 2 h. The relative decrease in the concentration of arsenic in the effluent is given in the Table 1. The improved adsorption performance of CH-SA-CZ over CH-SA can be explained on the basis of the fact that the CuO and ZnO enhance the active adsorption surface area which in turn facilitates the surface complexation process of As on the metal oxide adsorption surface [25]. Moreover, CuO can trigger the decrease in As concentration through precipitation of Cu3AsO4 at the metal surface and the precipitate is adsorbed inside the cryogel’s spongy and porous scaffold the cryogel’s spongy and porous scaffold [32]. To establish our hypothesis, we have performed SEM, XRD and FTIR of the As adsorbed cryogel.
6.2 Adsorption studies
To explain the sorption efficiency, time-dependent batch adsorption of arsenic onto CH-SA-CZ adsorbent is studied (Fig. 5a). It is noted that initially the adsorption is fast due to the adsorption of ions onto the adsorbent surface. Then it slowly diffuses inside the pores and reach equilibrium. As a result there is a decrease in the rate of adsorption [25].
The kinetics of arsenic adsorption onto CH-SA-CZ cryogel surface follows pseudo-second order rate equation,
where K2 (g mg−1 min−1) represent the rate constant, and qe (mg/g) and qt (mg/g) are the equilibrium adsorption capacity and amount of arsenic adsorbed onto the surface at time t, respectively. The value of K2 was calculated from the slope of the graph between t/qt vs. t (Fig. 5b) and was found out to be 0.00936 g mg−1 min−1. Similarly, the value of qe is 2.429 mg/g as calculated from the intercept of the plot.
The maximum adsorption capacity of CH-SA-CZ cryogel and the type of adsorption were determined from Langmuir and Freundlich isotherm models. Monolayer deposition of adsorbate is described on the basis of Langmuir adsorption, whereas multilayer adsorption is explained by Freundlich isotherm model and the equation is given by [33]:
where Kf (mg/g) denotes the adsorption capacity and n is the adsorption intensity. For good physical adsorption, n > 1.
The experimental data of this study fit well into Freundlich isotherm model (Fig. 6) rather than Langmuir model (Figure S4) with correlation coefficient value (R2) around 0.91. By plotting Ce/qe against Ce over a range of arsenic concentration, a linear form of graph for Freundlich adsorption is obtained. The adsorption capacity, Kf is obtained from the intercept of the graph and the value is found to be 0.899 mg/g. The value of n, adsorption intensity is found to be 1.278 as calculated from the slope of the graph.
As shown in Fig. 7b, the arsenic loaded SEM micrograph of CH-SA-CZ clearly depicts that almost no pores are visible on the metal oxide doped cryogel matrix after arsenic loading. EDX spectra confirm the presence of arsenic on the adsorbent (Figure S1).
Similar confirmation is observed in Powder XRD pattern of CH-SA-CZ after arsenic adsorption. Few additional peaks of As (2θ = 32.03°, 34.64°) were identified along with the diffraction peaks of CuO and ZnO. The residual crystallinity of a polymer is responsible for controlling the accessibility of metal ions to the sorption sites. All the free –NH2 groups are not available as active binding sites due to some intra or intermolecular hydrogen bond. As crystallinity of the polymer increases, the degree of accessibility of metal ions to the amine group decreases correspondingly [29]. Figure 8a shows that the diffraction intensity of CH-SA-CZ is stronger than that for CH-SA at 2θ = 22.3° implying an increase in the degree of crystallinity of the material after arsenic adsorption. A higher crystallinity implies that the number of metal binding sites has been decreased due to a lesser number of the available functional group [20].
FTIR images of arsenic loaded CH-SA-CZ as shown in Fig. 8b implies that the intensity of transmission are less in the arsenic loaded CH-SA-CZ than CH-SA-CZ due to pollutant adsorption. This phenomenon may be attributed to the presence of higher density of functional groups in the Cu and Zn doped CH-SA material. As a result it provides more number of binding sites to adsorb metal ions. After arsenic adsorption there is significant decrease in accessible amine groups [34].
In order to measure the recyclability, the studied chitosan cryogel is regenerated by dipping the matrix into acetone solvent for 30 min followed by washing with DI water. The washed regenerated matrix is then dried at 100 °C for 6 h. The dried regenerated chitosan matrix is then dipped into standard arsenic solution to perform adsorption studies and the resulted effluents are investigated by means of ICPMS. The regeneration test is performed twice for CH-SA-CZ matrix. The effluents from the first and second cycles of regeneration process are studied in ICPMS. From Fig. 9, it is confirmed that the regeneration capacity of the material is decreasing as there is a gradual increase in arsenic concentration in consecutive cycles that means after regeneration with acetone the scope of reusability of the material is reduced.
7 Conclusion
The present study explicitly demonstrates the superiority of CH-SA-CZ sorbent for As removal in drinking water. It interacts with the metal anions through anion exchange/electrostatic attraction. The synthesized metal oxide doped cross-linked chitosan was completely characterized by SEM, XRD, EDAX and FTIR. The arsenic contamination is reduced to 76% after 2 h of contact time with only 10 mg of adsorbent. But, in order to reach the target decontamination level or to achieve maximum sorption capacity, sorbent dosage may be adjusted. The mechanism of As removal was established with different characterization techniques like SEM, XRD and FTIR. The adsorbent can be regenerated with acetone treatment. The high removal efficiency of arsenic metal ion and recyclability of the CuO–ZnO doped cross linked chitosan render these biopolymers derived cryogel a potential candidate for low cost arsenic removal filter development.
References
Elwakeel KZ, Guibal E (2015) Arsenic (V) sorption using chitosan/Cu(OH)2 and chitosan/CuO composite sorbents. Carbohydratepolymers 134:190–204
Jain CK, Ali I (2000) Arsenic: occurrence, toxicity and speciation techniques. Water Res 34:4304–4312
Jiang JQ, Ashekuzzaman S, Jiang A, Sharifuzzaman S, Chowdhury S (2013) Arsenic contaminated groundwater and its treatment options in Bangladesh. Int J Environ Res Public Health 10(1):18–46
Razmgar K, Saljoughi E, Mousavi SM (2019) Preparation and characterization of a novel hydrophilic PVDF/PVA/Al2O3 nanocomposite membrane for removal of As(V) from aqueous solutions. Polym Compos 40(6):2452–2461
Nitzsche KS, Lan VM, Trang PTK, Viet PH, Berg M, Voegelin A, Schröder C (2015) Arsenic removal from drinking water by a household sand filter in Vietnam—effect of filter usage practices on arsenic removal efficiency and microbiological water quality. Sci Total Environ 502:526–536
Wang Y, Duan J, Liu S, Li W, van Leeuwen J, Mulcahy D (2014) Removal of As(III) and As(V) by ferric salts coagulation–implications of particle size and zeta potential of precipitates. Sep Purif Technol 135:64–71
Hokkanen S, Repo E, Lou S, Sillanpää M (2015) Removal of arsenic(V) by magnetic nanoparticle activated microfibrillated cellulose. Chem Eng J 260:886–894
Martinez-Vargas S, Martínez AI, Hernández-Beteta EE, Mijangos-Ricardez OF, Vázquez-Hipólito V, Patiño-Carachure C, Lopez-Luna J (2017) Arsenic adsorption on cobalt and manganese ferrite nanoparticles. J Mater Sci 52(11):6205–6215
Maher A, Sadeghi M, Moheb A (2014) Heavy metal elimination from drinking water using nanofiltration membrane technology and process optimization using response surface methodology. Desalination 352:166–173
Sato Y, Kang M, Kamei T, Magara Y (2002) Performance of nanofiltration for arsenic removal. Water Res 36:3371–3377
Kim J, Benjamin MM (2004) Modelling a novel ion exchange process for arsenic and nitrate removal. Water Res 38:2053–2062
Akin I, Arslan G, Tor A, Cengeloglu Y, Ersoz M (2011) Removal of arsenate [As(V)] and arsenite [As(III)] from water by SWHR and BW-30 reverse osmosis. Desalination 281:88–92
Paul B, Parashar V, Mishra A (2015) Graphene in the Fe 3 O 4 nano-composite switching the negative influence of humic acid coating into an enhancing effect in the removal of arsenic from water. Environ Sci Water Res Technol 1(1):77–83
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112(10):5073–5091
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308:438–462
Ranjbar E, Ghiassi R, Akbary Z (2017) Lead removal from groundwater by granular mixtures of pumice, perlite and lime using permeable reactive barriers. Water Environ J 31(1):39–46
Cheung R, Ng T, Wong J, Chan W (2015) Chitosan: an update on potential biomedical and pharmaceutical applications. Marinedrugs 13(8):5156–5186
Zhang L, Zeng Y, Cheng Z (2016) Removal of heavy metal ions using chitosan and modified chitosan: a review. J Mol Liq 214:175–191
Satapathi S (2017) Graphene-based 3D xerogel as adsorbent for removal of heavy metal ions from industrial wastewater. J Renew Mater 5(2):96–102
Peng X, Chen J, Kong L, Hu X (2018) Removal of arsenic from strongly acidic wastewater using phosphorus pentasulfide as precipitant: UV-light promoted sulfuration reaction and particle aggregation. Environ Sci Technol 52(8):4794–4801
Gupta A, Yunus M, Sankararamakrishnan N (2013) Chitosan-and iron–chitosan-coated sand filters: a cost-effective approach for enhanced arsenic removal. Ind Eng Chem Res 52(5):2066–2072
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38:43–74
Mitra T, Sailakshmi G, Gnanamani A, Mandal A (2013) Studies on cross-linking of succinic acid with chitosan/collagen. Mater Res 16(4):755–765
Piron E, Domard A (1998) Interaction between chitosan and uranyl ions. Part 2. Mechanism of interaction. Int J Biol Macromol 22(1):33–40
Malwal D, Gopinath P (2016) Rapid and efficient removal of arsenic from water using electrospun CuO–ZnO composite nanofibers. RSC Adv 6(116):115021–115028
Dimapilis EAS, Hsu CS, Mendoza RMO, Lu MC (2018) Zinc oxide nanoparticles for water disinfection. Sustain Environ Res 28(2):47–56
Miller SM, Zimmerman JB (2010) Novel, bio-based, photoactive arsenic sorbent: TiO2-impregnated chitosan bead. Water Res 44(19):5722–5729
Beker U, Cumbal L, Duranoglu D, Kucuk I, Sengupta AK (2010) Preparation of Fe oxide nanoparticles for environmental applications: arsenic removal. Environ Geochem Health 32(4):291–296
Chang YN, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5(12):2850–2871
Kumar PS, Selvakumar M, Babu SG, Jaganathan SK, Karuthapandian S, Chattopadhyay S (2015) Novel CuO/chitosan nanocomposite thin film: facile hand-picking recoverable, efficient and reusable heterogeneous photocatalyst. RSC Adv 5(71):57493–57501
Ou CY, Li SD, Li CP, Zhang CH, Yang L, Chen CP (2008) Effect of cupric ion on thermal degradation of chitosan. J Appl Polym Sci 109(2):957–962
Liu G, Talley JW, Na C, Larson SL, Wolfe LG (2010) Copper doping improves hydroxyapatite sorption for arsenate in simulated groundwaters. Environ Sci Technol 44(4):1366–1372
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156(1):2–10
Boddu VM, Abburi K, Talbott JL, Smith ED, Haasch R (2008) Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Res 42(3):633–642
Acknowledgements
SS like to acknowledge DST Water Technology Initiative Grant [No. DST/TM/WTI/2K16/50(G)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Purohit, S., Chini, M.K., Chakraborty, T. et al. Rapid removal of arsenic from water using metal oxide doped recyclable cross-linked chitosan cryogel. SN Appl. Sci. 2, 768 (2020). https://doi.org/10.1007/s42452-020-2525-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2525-6