Abstract
Two compounds, having dimethoxy-substituted triphenylamino groups and fragments of cyanoacrylic acid or rhodanine-3-acetic acid were prepared and characterized. Their optical, photophysical, thermal, electrochemical, photoelectrical and nonlinear optical properties were investigated. Both derivatives showed ability of glass-formation with glass transition temperatures of 83 and 61 °C. They showed comparable ionization potential values of ca. 5.20 eV. The compounds showed Kerr and two photon absorption effects. The dye containing a rhodanine-3-acetic acid fragment, namely 2-((E)-5-(4-(diphenylamino)benzylidene)-tetrahydro-4-oxo-2-thioxothiophen-3-yl)acetic acid, exhibited a promising power conversion efficiency of 2.09% in dye-sensitized solar cells using the spiro-OMeTAD as hole transporting compound.
Similar content being viewed by others
1 Introduction
In recent years, the investigation on solar cells using organic semiconducting derivatives has made considerable advance [1,2,3]. Among the promising photovoltaic technologies, dye-sensitized solar cells (DSSCs) have received much attention recently due to their low cost and relatively high efficiency [4,5,6]. Generally, DSSCs are based on a photoelectrode sensitized by a dye [7,8,9], where photo-induced electron transfers occur [7,8,9]. A counter electrode and a liquid electrolyte complete the cell [10, 11]. High efficiencies of ca. 13% were reached by employing a metal complex redox couple [namely Co(II/III)tris(bipyridyl)] and organic dyes [10, 11]. Hole transporting materials (HTMs) were successfully applied in solid state dye-sensitized solar cells (ssDSSCs) which allow to avoid potential leakage problems associated with the volatile nature of the liquid electrolyte [12, 13]. During the last few years, spiro-OMeTAD has shown ascending performance when related with organic dyes such as Y123 (7.2%) [14] and D102 (4.2%) [15]. However, the synthesis of these dyes requires a multistep procedures which are costly and time consuming [16].
Most conventional organic dyes possess the push–pull structure of “donor-conjugated bridge-acceptor”. Typically, organic dyes used for efficient solar cells are required to afford intense and broad absorption in the visible spectral region. Aside from donor units, the electron acceptor plays a significant role in the performance control of DSSCs [17].
Recently, derivatives containing triphenylamino moieties as donor units have shown potential applications in photovoltaic devices [17]. Triphenylamino moiety is able to extend the light absorption capability of the dyes [18, 19]. Moreover, triphenylamine is sustained as a perfect donor moiety in organic dyes, since it is able to demonstrate high hole-transporting and a perfect electron-donating capability [20]. Introducing an additional electron-donating moiety into derivative of triphenylamine can enhance charge separation, which result in high overall conversion efficiency [21]. Due to the certain peculiarities of the structure of triphenylamine, electron acceptor groups can be introduced. They can affect the molar extinction coefficients, absorption spectra, and the HOMO, LUMO energy levels of the derivatives. Rhodanine 3-acetic acid and cyanoacetic acid were introduced into triphenylamine-based dyes as acceptor moieties [22, 23].
As demand for new nonlinear optical (NLO) materials for optical data storage [24], optical data processing [25] and other NLO applications grows, more and more researches are dedicated to study NLO properties of different materials. Organic NLO materials have attracted great interest due to possibility to tune NLO properties by varying molecular structure. Although vast number of papers has already been published regarding structure-properties relations of organic NLO materials [26,27,28], still there is lack of qualitative criteria to theoretically design organic molecules for NLO applications.
On the other hand, in order to further improve the characteristics of triphenylamine sensitized solar cells, deeper insight should be committed to the structure–property relationships of triphenylamine-based of dyes. In this paper, easily synthesized dyes based on dimethoxy-substituted triphenylamines were obtained by a Knoevenagel condensation. Two different dyes containing cyanoacrylic acid (compound 1) and rhodanine-3-acetic acid (compound 2) moieties as an electron acceptors were prepared. These dyes show interesting electronic and optoelectronic properties and, as we show, constitute potential candidates to be used in solid state dye sensitized solar cells based using the structure of FTO/TiO2/Dye/Spiro-OMeTAD/Ag. In addition, we studied Kerr and two-photon absorption effects of the synthesized derivatives to understand how different acceptor groups influence NLO properties.
2 Experimental section
2.1 Reagents
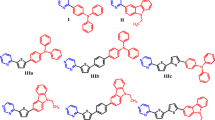
Cyanoacetic acid, rhodanine-3-acetic acid, zinc acetate dihydrate (Zn(CH3COO)2·2H2O) were purchased from Sigma Aldrich. The starting compounds 4-methoxy-N-(4-methoxyphenyl)-N-phenylaniline (I, m.p. = 104–105 °C, lit. [29] m.p. = 104–105 °C), 4-[bis(4-methoxyphenyl)amino]benzaldehyde (II, m.p. = 90–92 °C, lit. [30] m.p. = 92–93 °C) were synthesized by the previous described procedures.
2.2 Instrumentation
13C NMR, 1H NMR spectra were obtained using a Varrian Unity Inova (700 MHz (1H) and 176 MHz (13C)). Mass (MS) spectra, infrared (IR) spectra were recorded, elemental, thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) measurements, absorption, photoluminescence (PL) spectra of dilute solutions and of the films, cyclic voltammetry (CV) measurements were recorded as described earlier [31, 32]. UV–Vis spectra of dilute solutions of the compounds were recorded in quartz cells by Perkin Elmer Lambda 35 spectrometer. Photoluminescence (PL) spectra of dilute solutions of the compounds were recorded using Edinburgh Instruments’ FLS980 Fluorescence Spectrometer. Ionization potential measurements of the solid samples were performed by photoelectron emission method in air [33, 34]. Hole mobilities (μh) were studied by time of flight technique [35, 36]. Kerr and TPA effects we implemented the Z-scan method. Experimental measurements were carried out using 1064 nm Nd:YAG laser with 30 ps pulse duration and 10 Hz pulse repetition rate. The current density–voltage (J–V) characteristics of the cells were recorded in air using a Keithley 2400 source-measure unit, in the dark and under simulated solar emission (Atlas Solarconstant 575PV). The spectral mismatch between the emission of the solar simulator and the global AM1.5G solar spectrum (IEC 60904-3 Ed.2) was corrected using a mismatch factor and the solar simulator irradiance was adjusted accordingly using a certified silicon reference cell in order to achieve an equivalent AM1.5G irradiance of 1 sun (100 mW cm−2) on the tested cells.
3 Results and discussion
3.1 Synthesis
The intermediate derivative (II) was prepared by formylation reaction of the derivative of triphenylamine (I). Compounds 1 and 2 were synthesyzed by Knoevenagel condensation of the formyl derivative of triphenylamine (II) with cyanoacrylic acid or rhodanine-3-acetic acid, respectively in DMF using zinc acetate dihydrate (Scheme 1). The chemical structures of 1 and 2 were proved by IR, 1H NMR and 13C NMR spectroscopies, mass spectrometry, elemental analysis. Derivatives are soluble in acetone, dichloromethane, THF, ethylacetate etc.
3.2 Thermal properties
The thermal characteristics of 1 and 2 were obtained by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). Derivatives 1, 2 were obtained after the synthesis and purification as crystalline substances as confirmed by DSC. The DSC thermograms of 1 and 2 are shown in Figs. 1, S1. When the crystalline samples were heated in the first heating scan, the endothermic peaks due to melting were observed at 201 and 121 °C, respectively. When the melted samples were cooled down and heated again, glass transition temperatures (Tg) were observed at 83 °C (for 1) or 61 °C (for 2). Low molar mass compounds with stable amorphous phase above room temperature are named as molecular glasses [37]. They are widely used in modern devices of organic optoelectronics. Indeed, in dye-sensitized solar cells, Tg should not be too low, to prevent the softening of the HTMs during illumination. The standard HTM material is spiro-OMeTAD and its Tg is 120 °C [38]. The higher Tg is more favorable for the encapsulation process of a cell. In our case, Tg of dye 1 having cyanoacrylic acid group is by 22 °C higher than that of dye 2 containing rhodanine-3-acetic acid moiety (83 °C vs. 61 °C). This observation can apparently be explained by the stronger intermolecular interaction in the solid sample of 1. Tg can be controlled by the grafting of substituents on to the 4 position of the 4-methoxy-N-(4-methoxyphenyl)-N-phenylaniline moiety [29, 39]. Attachment of bulky substituents results in the increase of molecular weight and the rigidity of the compounds and thus increase their Tg [40,41,42,43,44,45].
TGA revealed single-stage decomposition of compounds 1, 2 (Fig. S1). Compound 1 showed considerably higher 5% weight loss temperature of 283 °C as compared with that of compound 1 (195 °C, Table 1). This observation can apparently be explained by the lower thermal stability of the rhodanine-3-acetic acid moiety relative to that of the fragment of cyanoacrylic acid. Higher thermal stability of compound 1 can also be explained by the stronger intermolecular interaction in the solid state, which is evidenced by considerably higher melting point.
3.3 Optical properties
UV absorption spectra of the dilute solutions of 1 and 2 in toluene (10−5 M) are shown in Fig. 2.
Absorption intensity maxima at 424 and 466 nm observed in UV spectra of the solutions of 1 and 2 can be assigned to an intramolecular charge transfer (ICT) between cyanoacrylic acid or rhodanine-3-acetic acid moieties and triphenylamino unit [46, 47]. UV spectrum of the solution of compound 2 exhibited red-shift with respect of that of the solution of compound 1. The solution of compound 1 having cyanoacrylic acid moiety exhibited higher molar extinction coefficients at the maximum absorption wavelength compared to those of compound 2 containing rhodanine-3-acetic acid fragment. The relatively high molar extinction coefficient of the solution of 1 (2.4 × 104 M−1 cm−1) indicates its good ability for light harvesting. The optical band gap (E gopt ) of the solution of 1 (2.28 eV) was found to be lower than that of the solution of 2 (2.52 eV, Table 2).
The wavelengths of emission intensity maxima of the solutions of 1 and 2 were found to be close (552 and 553 nm, respectively). The Stokes shift of the solution of compound 1 (128 nm) was found to be considerably larger than that of the solution of 2 (87 nm). This observation can be attributed to the geometrically more relaxed structure of derivative 1 upon excitation [48]. The solutions of materials 1 and 2 showed relatively low fluorescence quantum yields (19.56 and 14.25%, respectively).
3.4 Frontier orbitals, photoelectrical and electrochemical properties
Electrochemical properties of derivatives 1 and 2 were investigated by cyclic voltammetry (CV) using their 1 × 10−6 M solutions in dichloromethane. CVs of the derivatives are present in Figs. 3a, S2. The onset oxidation potential vs. Fc together with the values of the electron affinity (EACV) and ionization potential (IPCV) values are listed in Table 3.
Oxidation of 1 and 2 is apparently related to the loss of electrons of TPA moiety, resulting in radical cations (one redox system) [49]. No reduction potentials were detected. Compound 1 displayed a reversible oxidation potential in positive potential range at 0.43 V, whereas 2 showed a quasi-reversible oxidation potential at 0.38 V versus Fc. The presence of cyanoacrylic acid moiety in compound 1 resulted in slightly higher oxidation potential and thus slightly higher IPCV value. The calculated EACV values for derivatives 1 and 2 were found to be − 2.91 and − 2.70 eV, respectively. In compound 1 the acceptor moiety apparently has stronger influence on the electrochemical properties as compared to 2. For this reason, 1 showed higher IPCV values than compound 2. These results are in agreement with the results of density functional theory (DFT) calculations. The low energy absorption band of 1 appeared at lower energy than that of 2.
Differential pulse voltammperometric (DPV [50]) spectra (Fig. 3b) of compounds 1 and 2 showed peaks of oxidation processes. The oxidation potential peak (0.87 V) of 1 having cyanoacrylic acid moiety was observed at a slightly higher potential compared to that of compound 2 containing rhodanine-3-acetic acid moiety (0.96 V).
To investigate electron distribution of the compounds, their structures were optimized using DFT calculations with Spartan 14 program [51]. The calculations were performed with the B3LYP under 6-31G (d) basis set. Figure 4 presents the frontier molecular orbitals of the compounds.
For both 1 and 2, HOMOs are distributed over the whole molecular structures. LUMO of 1 and 2 are mainly concentrated on cyanoacrylic acid and rhodanine moieties, especially on carbonyl and thiocarbonyl groups, as well as on the neighbouring benzene rings. The energies of HOMO and LUMO for 1 were found to be − 5.15 and − 2.12 eV, respectively, and those estimated for 2 were − 5.02 and − 2.48 eV, respectively. It was found that the calculated values are in good agreement with the experimental CV data.
Ionization potential values of thin solid layers (IPPE) of derivatives 1, 2 were estimated by photoelectron emission spectrometry. Photoelectron emission spectra of the solid layers of 1, 2 are depicted in Fig. 5. The IPPE values of the solid samples of 1 and 2 were found to be comparable (5.26 and 5.29 eV, respectively).
Charge-transporting properties of the layers of compound 1 were studied by time-of-flight (TOF) technique. To prepare the sample with the structure of ITO/organic layer/Al, the layer of compound 1 the sample was deposited by thermovacuum method. It was not possible to prepare the sample of compound 2 for the TOF measurements by the same method, apparently due to its relatively low thermal stability (Table 1). Hole drift mobility of 3.4 × 10−6 cm2/Vs at electric field of 5.2 × 105 V/cm was obtained for compound 1. Hole mobilities of the layer of 1 were found to be strongly dependent upon electric field. This dependence could be well described by a Poole–Frenkel type mobility using formula \(\mu = \mu_{0} e^{\alpha \sqrt E }\) [52]. Zero-field mobilities (µ0) and field dependence parameter (α) are given in Fig. 6. Relatively high dependence of hole mobilities versus electric field for derivative 1 can apparently be explained by the dispersive hole transport which is betrayed by TOF transients shown in the insert of Fig. 6.
3.5 NLO properties
Kerr and two-photon absorption effects describe materials refractive index and absorption changes, respectively, due to optical irradiance:
where n0 is linear refractive index, α0—linear absorption coefficient, n2—Kerr coefficient, α2 is TPA coefficient and I is optical intensity.
We studied Kerr and two-photon absorption effects of selected molecules to better understand how different acceptor groups influence third-order NLO properties. Organic molecules were dissolved in chloroform and contained in 2 mm thick optical quartz cells.
To study Kerr and two-photon absorption effects we implemented the Z-scan method. Details regarding experimental setup can be found in Ref. [53]. Laser beam was focused using lens with 11 cm focal length. Light transmitted through sample was measured simultaneously with open-aperture detector and closed-aperture detector with 1 mm aperture separating less than 1% of incident light. Laser beam waist radius at focus was calculated to be w0 = 26 μm. This allowed to employ thin-sample approximation for data fitting [54]. To acquire Kerr and two-photon absorption coefficients from experimental data we employed analytical expressions derived using Gaussian decomposition method [54]. During Z-scan experiment we measured how materials transmittance changes due to optical irradiance. For Kerr effect transmittance can be expressed as:
where z—sample position, z0—Rayleigh length and ∆Φ is phase change defined as:
where k—wave number and Leff—effective sample length defined as:
In case of TPA, transmittance of measured medium can be expressed as:
where q is TPA amplitude defined as:
While TPA coefficient allows us to compare different NLO sample, it does not allow us to compare molecules. For this we calculated two-photon absorption cross-section values that characterize single molecule contribution to two-photon absorption effect. Two-photon absorption cross-section can be calculated using the following equation [55]:
where hν is photon energy and N molecule concentration per cm3. To use analytical Eqs. (3) and (6) NLO medium needs to fit weak nonlinear model for which two conditions need to be satisfied—phase change |∆Φ| < π and two-photon absorption amplitude |q| < 1 [54].
Example for compound 1 experimental data is shown in Fig. 7. Phase changes for different concentration samples in case of 1 are shown in Fig. 8. It is evident that by increasing compound 1 concentration the phase changes decrease. This leads to conclusion that compound 1 possess negative Kerr coefficient. Acquired values for NLO coefficients for all molecules are listed in Table 4.
Real and imaginary parts of third-order susceptibility can be defined as [56]:
where λ—laser wavelength, ε0—vacuum dielectric constant, n0—refractive index an c—speed of light. Additionally, we carried out quantum chemical calculations of second-order hyperpolarizability real part γA using CPKS method in Gaussian 9. Further information about calculation parameters can be found in [53]. Experimental values for second-order hyperpolarizability were calculated using Eq. (11) and presented alongside third-order susceptibility values in Table 5 [57].
From all of the molecules only compound 1 possesses two-photon absorption coefficient due to considerable absorption at 532 nm. By comparing the values of Kerr coefficient it is evident that by adding a stronger acceptor group we increase the magnitude of Kerr effect. Experimental and quantum calculation values for second-order hyperpolarizability are shown in Fig. 9.
Experimental and quantum calculation values of second-order hyperpolarizability. Blue point indicate molecules studied in this paper while red dot indicates triphenylamine studied in our previous paper [30]
It is evident that derivatives I and II gives a similar slope values as other derivatives of triphenylamine reported in other papers [30, 53]. At the same time, derivatives 1 and 2 displays much larger difference between experimental and quantum calculation values. We speculate that this is due to aggregation processes that can lead to larger third-order NLO values. Similar enhancement of NLO properties due to aggregation has been previously reported in literature [58].
3.6 Photovoltaic properties
The photovoltaic properties of compounds 1 and 2, used as dyes, were investigated by fabricating solid-state dye sensitized solar cells with spiro-OMeTAD as p-type organic hole transporting material (HTM). The device structure was FTO/TiO2/Dye/Spiro-OMeTAD/Ag. Knowing the IP values of 5.23 and 5.18 eV and the EA values of 2.91 and 2.70 eV, respectively for 1 and 2, we can expect that these dyes would be suitable (1) for electron transfer to the TiO2 nanostructured electrode as well as (2) to be regenerated by HTM. In comparison, IP and EA values of D102 are 5.32 and 3.38 eV respectively [59]. EA values of the new dyes are expected to prevent any electron transfer from the dye to the LUMO of the spiro-OMeTAD (EA value of the HTM is 2.05 eV) [60]. In summary, the energy levels of the synthesized dyes are clearly well suited for FTO/TiO2/Dye/Spiro-OMeTAD/Ag device.
Usually, the solvent used to prepare the solution of D102 is a mixture of acetonitrile and tert-butanol (ACN/tert-BuOH). The solution of dye 1 has been prepared and deposited under these conditions. Nevertheless, dye 2 was found to be hardly soluble in this solvent mixture. That is why THF as solvent was preferred for dye 2. The same dye concentration (0.06 mM) was chosen for both compounds 1 and 2.
Figure 10a shows photocurrent–voltage curves and IPCE spectra of 1- and 2-sensitized DSSCs. Photovoltaic parameters are summarized in Table 6. Photocurrent of the 2-sensitized DSSC is higher than that of the 1-sensitized one, which is due to more delocalized electron distribution in the HOMO state (see Fig. 4). The open-circuit voltage (VOC) are of ca. 0.65 V for both dyes. The higher VOC (0.80 V) observed for the organic D102 dye (Table S1) is probably related to the recombination kinetics at the TiO2/Dye/HTM interface. Indeed, the energy offset for electron injection from the LUMO level of the dye to the TiO2 conduction band is found to be much larger for compounds 1 and 2 compared to D102, which can explain a substantial energy loss of the photo-excited electrons. Moreover, the energy difference between the LUMO of compound 2 (and in less extend of compound 1) and LUMO level of spiro-OMeTAD is largely reduced compared to D102 (Fig. S3). The energy barrier for direct electron injection to spiro-OMeTAD is therefore reduced, which can favor charge recombination and prevent efficient charge injection. Such phenomenon is in agreement with the larger dark current observed under reverse applied voltage (see Fig. S4). Finally, although the power conversion efficiency of the devices with dye 1 is quite low (1.02%), the devices built with dye 2 show interesting PCE (2.09%), close to that D102 reference (2.47%). The limited PCE for the devices built with dye 1 is most likely due to the blue-shift in optical absorption, which is less favorable for efficient charge generation. Since the maximum of absorption of 1 is shifted to lower wavelengths compared to those of D102 and 2, we can observe the beginning of a screen effect (overlapping of both dye and HTM absorption). Although several devices have been prepared (Fig. S5) and even if the PCE are lower than that of the D102 reference dye without further optimizations (i.e. in concentration), the results show a promising tendency for both dyes.
Figure 10b shows the external quantum efficiency (EQE) spectra of the solid-state DSSC [FTO/TiO2/Dye/Spiro-OMeTAD/Ag] containing 1 and 2, recorded under the monochromatic illumination. The devices based on 2 show a broad response which extends up to 650 nm, while compound 1 only exploits incoming photons up to 520 nm. The IPCE spectra are in agreement with the optical absorption data, where the maximum absorption of dye 1 is blue-shifted leading to an increased screening effect of the solar light. IPCE shows clearly that dye 2 is better suited in terms of absorption for solid-state DSSC.
4 Conclusions
Dimethoxy-substituted triphenylamine derivatives containing cyanoacrylic acid and rhodanine-3-acetic acid moieties were synthesized by Knoevenagel condensation. The target derivatives form molecular glasses with glass transition temperatures of 83 and 61 °C. They show moderate thermal stability with the temperatures of degradation onsets of 283 and 195 °C. The cyclic voltammetry measurements revealed close ionization potential (5.23 and 5.18 eV) and electron affinity values (− 2.91 and − 2.70 eV) in the solid state. The layer of derivative containing cyanoacrylic acid moiety showed time-of-flight hole drift mobility of 3.4 × 10−6 cm2/Vs at electric field of 5.2 × 105 V/cm. This compound also showed two-photon absorption effect as it was the only compound exhibiting considerable absorption at 532 nm. Compounds containing acceptor groups possessed larger nonlinear refractive index values than dimethoxy substituted triphenylamines. Dye sensitized solar cells with the structure FTO/TiO2/Dye/Spiro-OMeTAD/Ag were fabricated using the synthesized compounds as dyes. The photovoltaic properties of the devices were found to be in agreement with the electronic and optical properties. The dye containing a rhodanine-3-acetic acid moiety exhibited a promising power conversion efficiency of 2.09% in solid state dye sensitized solar cell using the spiro-OMeTAD as hole transporting compound.
References
Jung JW, Jo JW, Jung EH et al (2016) Recent progress in high efficiency polymer solar cells by rational design and energy level tuning of low bandgap copolymers with various electron-withdrawing units. Org Electron 31:149–170
Kahle FJ, Saller C, Köhler A et al (2017) Crosslinked semiconductor polymers for photovoltaic applications. Adv Energy Mater 7:1700306–1700315
Lincker F, Delbosc N, Bailly S et al (2008) Fluorenone-based molecules for bulk-heterojunction solar cells: synthesis, characterization, and photovoltaic properties. Adv Funct Mater 18:3444–3453
Grätzel M, O’Regan B (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Ning Z, Fu Y, Tian H (2010) Improvement of dye-sensitized solar cells: what we know and what we need to know. Energy Environ Sci 3:1170–1181
Ning Z, Tian H (2009) Triarylamine: a promising core unit for efficient photovoltaic materials. Chem Commun 45:5483–5495
Hagfeldt A, Cappel UB, Boschloo G et al (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663
Tian HN, Sun LC (2011) Iodine-free redox couples for dye-sensitized solar cells. J Mater Chem 21:10592–10601
Wang M, Grätzel C, Zakeeruddin SM et al (2012) Recent developments in redox electrolytes for dye-sensitized solar cells. Energy Environ Sci 5:9394–9405
Yella A, Lee H, Tsao HN et al (2011) Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 334:629–633
Mathew S, Yella A, Gao P et al (2014) Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat Chem 6:242–247
Cells SDS, Leijtens T, Ding I et al (2012) Hole transport materials with low glass transition temperatures and high solubility for application in solid state DSC. ACS Nano 6:1455–1462
Liu P, Xu B, Karlsson KM et al (2015) The combination of a new organic D–π–A dye with different organic hole-transport materials for efficient solid-state dye-sensitized solar cells. J Mater Chem A 3:4420–4427
Burschka J, Dualeh A, Kessler F et al (2011) Tris(2-(1 H-pyrazol-1-yl)pyridine)cobalt(III) as p-type dopant for organic semiconductors and its application in highly efficient solid-state dye-sensitized solar cells. J Am Chem Soc 133:18042–18045
Melhem H, Simon P, Beouch L et al (2011) TiO2 nanocrystals synthesized by laser pyrolysis for the up-scaling of efficient solid-state dye-sensitized solar cells. Adv Energy Mater 1:908–916
Xu B, Bi D, Hua Y et al (2016) A low-cost spiro[fluorene-9,9′-xanthene]-based hole transport material for highly efficient solid-state dye-sensitized solar cells and perovskite solar cells. Energy Environ Sci 9:873–877
Zeng W, Cao Y, Bai Y et al (2010) Efficient dye-sensitized solar cells with an organic photosensitizer featuring orderly conjugated ethylenedioxythiophene and dithienosilole blocks. Chem Mater 22:1915–1925
Mahmood A (2016) Triphenylamine based dyes for dye sensitized solar cells: a review. Sol Energy 123:127–144
Yum JH, Hagberg DP, Moon SJ et al (2009) A light-resistant organic sensitizer for solar-cell applications. Angew Chem Int Ed 48:1576–1580
Wang C, Li J, Cai S et al (2012) Performance improvement of dye-sensitizing solar cell by semi-rigid triarylamine-based donors. Dyes Pigments 94:40–48
Zhang L, Liu Y, Wang Z et al (2010) Synthesis of sensitizers containing donor cascade of triarylamine and dimethylarylamine moieties for dye-sensitized solar cells. Tetrahedron 66:3318–3325
Abbotto A, Manfredi N, Marinzi C et al (2009) Di-branched di-anchoring organic dyes for dye-sensitized solar cells. Energy Environ Sci 2:1094–1101
Jiang X, Karlsson KM, Gabrielsson E et al (2011) Highly efficient solid-state dye-sensitized solar cells based on triphenylamine dyes. Adv Funct Mater 21:2944–2952
Hu D, Hu Y, Huang W et al (2012) Two-photon induced data storage in hydrogen bonded supramolecular azopolymers. Opt Commun 285:4941–4945
Ahadi S, Granpayeh N (2015) Femtosecond all-optical switching based on asymmetric plasmonic Kerr Fabry–Perot resonators. Opt Commun 349:36–41
Belfield KD, Morales AR, Kang BS et al (2004) Synthesis, characterization, and optical properties of new two-photon-absorbing fluorene derivatives. Chem Mater 16:4634–4641
Webster S, Fu J, Padilha LA et al (2008) Comparison of nonlinear absorption in three similar dyes: polymethine, squaraine and tetraone. Chem Phys 348:143–151
Padilha LA, Webster S, Przhonska OV et al (2009) Nonlinear absorption in a series of donor-π-acceptor cyanines with different conjugation lengths. J Mater Chem 19:7503–7513
Zilinskaite V, Gudeika D, Grazulevicius JV et al (2015) Derivatives of indandione and differently substituted triphenylamine with charge-transporting and NLO properties. Dyes Pigments 113:38–46
Gudeika D, Bundulis A, Mihailovs I et al (2017) Donor and acceptor substituted triphenylamines exhibiting bipolar charge-transporting and NLO properties. Dyes Pigments 140:431–440
Hladka I, Lytvyn R, Volyniuk D et al (2018) W-shaped bipolar derivatives of carbazole and oxadiazole with high triplet energies for electroluminescent devices. Dyes Pigments 149:812–821
Gudeika D, Sini G, Jankauskas V et al (2016) Synthesis and properties of the derivatives of triphenylamine and 1,8-naphthalimide with the olefinic linkages between chromophores. RSC Adv 6:2191–2201
Sych G, Simokaitiene J, Bezvikonnyi O et al (2018) Exciplex-enhanced singlet emission efficiency of nondoped organic light emitting diodes based on derivatives of tetrafluorophenylcarbazole and tri/tetraphenylethylene exhibiting aggregation-induced emission enhancement. J Phys Chem C 122:14827–14837
Butkute R, Lygaitis R, Mimaite V et al (2017) Bipolar highly solid-state luminescent phenanthroimidazole derivatives as materials for blue and white organic light emitting diodes exploiting either monomer, exciplex or electroplex emission. Dyes Pigments 146:425–437
Gudeika D, Reghu RR, Grazulevicius JV et al (2013) Electron-transporting naphthalimide-substituted derivatives of fluorene. Dyes Pigments 99:895–902
Ivanauskaite A, Lygaitis R, Raisys S et al (2017) Structure-property relationship of blue solid state emissive phenanthroimidazole derivatives. Phys Chem Chem Phys 19:16737–16748
Strohriegl P, Grazulevicius JV (2002) Charge-transporting molecular glasses. Adv Mater 14:1439–1452
Bach U, Lupo D, Comte P et al (1998) Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 395:583–585
Gudeika D, Grazulevicius JV, Sini G et al (2014) New derivatives of triphenylamine and naphthalimide as ambipolar organic semiconductors: experimental and theoretical approach. Dyes Pigments 106:58–70
Patel JP, Xiang ZG, Hsu SL et al (2015) Path to achieving molecular dispersion in a dense reactive mixture. J Polym Sci Polym Phys 53:1519–1526
Patel JP, Deshmukh S, Zhao C et al (2016) An analysis of the role of nonreactive plasticizers in the crosslinking reactions of a rigid resin. J Polym Sci Polym Phys 55:206–213
Patel JP, Zhao CX, Deshmukh S et al (2016) An analysis of the role of reactive plasticizers in the crosslinking reactions of a rigid resin. Polymer 107:12–18
Patel JP, Xiang ZG, Hsu SL et al (2017) Characterization of the crosslinking reaction in high performance adhesives. Int J Adhes Adhes 78:256–262
Patel JP, Hsu SL (2018) Development of low field NMR technique for analyzing segmental mobility of crosslinked polymers. J Polym Sci Pol Phys 56:639–643
Berton N, Nakar R, Schmaltz B (2019) DMPA-containing carbazole-based hole transporting materials for perovskite solar cells: Recent advances and perspectives. Synth Met 252:91–106
Xu W, Peng B, Chen J et al (2008) New triphenylamine-based dyes for dye-sensitized solar cells. J Phys Chem C 112:874–880
Roquet S, Cravino A, Leriche P et al (2006) Triphenylamine-thienylenevinylene hybrid systems with internal charge transfer as donor materials for heterojunction solar cells. J Am Chem Soc 128:3459–3466
Lai RY, Fabrizio EF, Lu L et al (2001) Synthesis, cyclic voltammetric studies, and electrogenerated chemiluminescence of a new donor—acceptor molecule: 3,7-[bis[4-phenyl-2-quinolyl]]-10-methylphenothiazine. J Am Chem Soc 123:9112–9118
Can M, Yigit MZ, Seintis K et al (2014) Synthesis of two tri-arylamine derivatives as sensitizers in dye-sensitized solar cells: electron injection studies and photovoltaic characterization. Synth Met 188:77–85
Drake KF, Van Duyne RP, Bond AM (1978) Cyclic differential pulse voltammetry: a versatile instrumental approach using a computerized system. J Electroanal Chem 89:231–246
Spartan’14 for Windows Version 1.1.2. 1840 Von Karman Avenue, Suite 370, Irvine, CA
Frenkel J (1938) On pre-breakdown phenomena in insulators and electronic semi-conductors. Phys Rev 54:647–648
Bundulis A, Nitiss E, Mihailovs I et al (2016) Study of structure-third-order susceptibility relation of indandione derivatives. J Phys Chem C 120:27515–27522
Sheik-Bahae M, Said AA, Wei TH et al (1990) Sensitive measurement of optical nonlinearities using a single beam. IEEE J Quantum Electron 26:760–769
Ajami A, Gruber P, Tromayer M et al (2015) Evidence of concentration dependence of the two-photon absorption cross section: determining the “true” cross section value. Opt Mater (Amst) 47:524–529
del Coso R, Solis J (2004) Relation between nonlinear refractive index and third-order susceptibility in absorbing media. J Opt Soc Am B 21:640
Sharafudeen KN, Adithya A, Vijayakumar S et al (2011) Multiphoton absorption process and self-focusing effect in coumarin derivative doped PMMA films by Z-scan and optical limiting studines. Curr Appl Phys 11:1089–1093
Yang L, Chen Z, Zhang S et al (2014) The tunable third-order optical nonlinearities of a diarylethene-zinc phthalocyanine hybrid. Dyes Pigments 102:251–256
Wang H, Liu G, Li X et al (2011) Highly efficient poly(3-hexylthiophene) based monolithic dye-sensitized solar cells with carbon counter electrode. Energy Environ Sci 4:2025–2029
Teh CH, Daik R, Lim EL et al (2016) A review of organic small molecule-based hole-transporting materials for meso-structured organic-inorganic perovskite solar cells. J Mater Chem A 4:15788–15822
Acknowledgements
This project has received funding from the Research Council of Lithuania (LMTLT), Agreement No. [S-LZ-19-2]. This research was funded by the Région Centre, the Tunisian ministry of research, University of Monastir and the French ministry of Higher Education and Research. J. Bouclé would like to thank the Sigma-Lim LabEx environment for financial supports, and the PLATINOM facility at XLIM laboratory regarding device fabrication and characterizations. DG acknowledges the Lithuanian Academy of Sciences for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gudeika, D., Bundulis, A., Benhattab, S. et al. Multifunctional derivatives of dimethoxy-substituted triphenylamine containing different acceptor moieties. SN Appl. Sci. 2, 327 (2020). https://doi.org/10.1007/s42452-020-2120-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2120-x