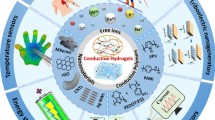
Abstract
Owing to their excellent mechanical flexibility, electrical conductivity, and biocompatibility, conductive hydrogels (CHs) are widely used in the fields of energy and power, and biomedical technology. To arrive at a better understanding of the design methods and development trends of CHs, this paper summarizes and analyzes related research published in recent years. First, we describe the properties and characteristics of CHs. Using Scopus, the world’s largest abstract and citation database, we conducted a quantitative analysis of the related literature from the past 15 years and summarized development trends in the field of CHs. Second, we describe the types of CH network crosslinking and basic functional design methods and summarize the three-dimensional (3D) structure-forming methods and conductive performance tests of CHs. In addition, we introduce applications of CHs in the fields of energy and power, biomedical technology, and others. Lastly, we discuss several problems in current CH research and introduce some prospects for the future development of CHs.
Graphic abstract













Similar content being viewed by others
References
Peng Q, Chen J, Wang T et al (2020) Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2(5):843–865. https://doi.org/10.1002/inf2.12113
El-Husseiny HM, Mady EA, Hamabe L et al (2022) Smart/stimuli-responsive hydrogels: cutting-edge platforms for tissue engineering and other biomedical applications. Mater Today Bio 13:100186. https://doi.org/10.1016/j.mtbio.2021.100186
Ying B, Liu X (2021) Skin-like hydrogel devices for wearable sensing, soft robotics and beyond. iScience 24(11):103174. https://doi.org/10.1016/j.isci.2021.103174
Chun KY, Seo S, Han CS (2022) Wearable all-gel multimodal cutaneous sensor enabling simultaneous single-site monitoring of cardiac-related biophysical signals. Adv Mater 34(16):2110082. https://doi.org/10.1002/adma.202110082
Su G, Zhang Y, Zhang X et al (2022) Soft yet tough: a mechanically and functionally tissue-like organohydrogel for sensitive soft electronics. Chem Mater 34(3):1392–1402. https://doi.org/10.1021/acs.chemmater.1c04211
Zhang J, Xin W, Qin Y et al (2022) “All-in-one” zwitterionic granular hydrogel bioink for stem cell spheroids production and 3D bioprinting. Chem Eng J 430:132713. https://doi.org/10.1016/j.cej.2021.132713
Huang Z, Chen X, O’Neill SJK et al (2022) Highly compressible glass-like supramolecular polymer networks. Nat Mater 21(1):103–109. https://doi.org/10.1038/s41563-021-01124-x
Xing ZG, Lin J, Zhao JW (2021) Overview of the artificial muscle actuators. J Mech Eng 57(9):1–11 (in Chinese). https://doi.org/10.3901/JME.2021.09.001
Lin Z, Jiang T, Shang J (2021) The emerging technology of biohybrid micro-robots: a review. Bio-Des Manuf 5(1):107–132. https://doi.org/10.1007/s42242-021-00135-6
Cheng T, Zhang YZ, Wang S et al (2021) Conductive hydrogel-based electrodes and electrolytes for stretchable and self-healable supercapacitors. Adv Funct Mater 31(24):2101303. https://doi.org/10.1002/adfm.202101303
Lu H, Zhang N, Ma M (2019) Electroconductive hydrogels for biomedical applications. WIREs Nanomed Nanobiotechnol 11(6):e1568. https://doi.org/10.1002/wnan.1568
Akter M, Bhattacharjee M, Dhar AK et al (2021) Cellulose-based hydrogels for wastewater treatment: a concise review. Gels 7(1):30. https://doi.org/10.3390/gels7010030
Alam A, Zhang Y, Kuan HC et al (2018) Polymer composite hydrogels containing carbon nanomaterials—morphology and mechanical and functional performance. Progr Polym Sci 77:1–18. https://doi.org/10.1016/j.progpolymsci.2017.09.001
Rong Q, Lei W, Liu M (2018) Conductive hydrogels as smart materials for flexible electronic devices. Chemistry 24(64):16930–16943. https://doi.org/10.1002/chem.201801302
English MA, Soenksen LR, Gayet RV et al (2019) Programmable CRISPR-responsive smart materials. Science 365(6455):780–785. https://doi.org/10.1126/science.aaw5122
Keplinger C, Sun JY, Foo CC et al (2013) Stretchable, transparent, ionic conductors. Science 341(6149):984–987. https://doi.org/10.1126/science.1240228
Deng Y, Huang M, Sun D et al (2018) Dual physically cross-linked κ-carrageenan-based double network hydrogels with superior self-healing performance for biomedical application. ACS Appl Mater Interf 10(43):37544–37554. https://doi.org/10.1021/acsami.8b15385
Stojkov G, Niyazov Z, Picchioni F et al (2021) Relationship between structure and rheology of hydrogels for various applications. Gels 7(4):255. https://doi.org/10.3390/gels7040255
Wang YJ, Zhang XN, Song Y et al (2019) Ultrastiff and tough supramolecular hydrogels with a dense and robust hydrogen bond network. Chem Mater 31(4):1430–1440. https://doi.org/10.1021/acs.chemmater.8b05262
Zhao D, Feng M, Zhang L et al (2021) Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohydr Polym 256:117580. https://doi.org/10.1016/j.carbpol.2020.117580
Palmese LL, Thapa RK, Sullivan MO et al (2019) Hybrid hydrogels for biomedical applications. Curr Opin Chem Eng 24:143–157. https://doi.org/10.1016/j.coche.2019.02.010
Chang B, Ahuja N, Ma C et al (2017) Injectable scaffolds: preparation and application in dental and craniofacial regeneration. Mater Sci Eng R Rep 111:1–26. https://doi.org/10.1016/j.mser.2016.11.001
Liu Q, Nian G, Yang C et al (2018) Bonding dissimilar polymer networks in various manufacturing processes. Nat Commun 9(1):846. https://doi.org/10.1038/s41467-018-03269-x
Li S, Wang L, Yu X et al (2018) Synthesis and characterization of a novel double cross-linked hydrogel based on Diels–Alder click reaction and coordination bonding. Mater Sci Eng C Mater Biol Appl 82:299–309. https://doi.org/10.1016/j.msec.2017.08.031
Arnfast L, Madsen CG, Jorgensen L et al (2014) Design and processing of nanogels as delivery systems for peptides and proteins. Ther Deliv 5(6):691–708. https://doi.org/10.4155/tde.14.38
Wei HL, Yao K, Yang Z et al (2011) Preparation of thermosensitive hydrogels by means of tandem physical and chemical crosslinking. Macromol Res 19(3):294–299. https://doi.org/10.1007/s13233-011-0308-z
Shao C, Chang H, Wang M et al (2017) High-strength, tough, and self-healing nanocomposite physical hydrogels based on the synergistic effects of dynamic hydrogen bond and dual coordination bonds. ACS Appl Mater Interf 9(34):28305–28318. https://doi.org/10.1021/acsami.7b09614
Mredha MTI, Pathak SK, Tran VT et al (2019) Hydrogels with superior mechanical properties from the synergistic effect in hydrophobic–hydrophilic copolymers. Chem Eng J 362:325–338. https://doi.org/10.1016/j.cej.2018.12.023
Oveissi F, Naficy S, Le TYL et al (2018) Tough and processable hydrogels based on lignin and hydrophilic polyurethane. ACS Appl Bio Mater 1(6):2073–2081. https://doi.org/10.1021/acsabm.8b00546
Deng Y, Hussain I, Kang M et al (2018) Self-recoverable and mechanical-reinforced hydrogel based on hydrophobic interaction with self-healable and conductive properties. Chem Eng J 353:900–910. https://doi.org/10.1016/j.cej.2018.07.187
Zhang XN, Wang YJ, Sun S et al (2018) A tough and stiff hydrogel with tunable water content and mechanical properties based on the synergistic effect of hydrogen bonding and hydrophobic interaction. Macromolecules 51(20):8136–8146. https://doi.org/10.1021/acs.macromol.8b01496
Chang X, Geng Y, Cao H et al (2018) Dual-crosslink physical hydrogels with high toughness based on synergistic hydrogen bonding and hydrophobic interactions. Macromol Rapid Commun 39(14):e1700806. https://doi.org/10.1002/marc.201700806
Liang Y, Xue J, Du B et al (2019) Ultrastiff, tough, and healable ionic-hydrogen bond cross-linked hydrogels and their uses as building blocks to construct complex hydrogel structures. ACS Appl Mater Interf 11(5):5441–5454. https://doi.org/10.1021/acsami.8b20520
Lin Y, Zhang H, Liao H et al (2019) A physically crosslinked, self-healing hydrogel electrolyte for nano-wire PANI flexible supercapacitors. Chem Eng J 367:139–148. https://doi.org/10.1016/j.cej.2019.02.064
Liu S, Oderinde O, Hussain I (2018) Dual ionic cross-linked double network hydrogel with self-healing, conductive, and force sensitive properties. Polymer 144:111–120. https://doi.org/10.1016/j.polymer.2018.01.046
Mondal S, Das S, Nandi AK (2020) A review on recent advances in polymer and peptide hydrogels. Soft Matter 16(6):1404–1454. https://doi.org/10.1039/c9sm02127b
Dai X, Zhang Y, Gao L et al (2015) A mechanically strong, highly stable, thermoplastic, and self-healable supramolecular polymer hydrogel. Adv Mater 27(23):3566–3571. https://doi.org/10.1002/adma.201500534
Guo Z, Zhang Z, Zhang N et al (2021) A Mg2+/polydopamine composite hydrogel for the acceleration of infected wound healing. Bioact Mater 15:203–213. https://doi.org/10.1016/j.bioactmat.2021.11.036
Zhang M, Yin Q, Ji X et al (2020) High and fast adsorption of Cd(II) and Pb(II) ions from aqueous solutions by a waste biomass based hydrogel. Sci Rep 10(1):3285. https://doi.org/10.1038/s41598-020-60160-w
Zhu H, Hu X, Liu B et al (2021) 3D printing of conductive hydrogel-elastomer hybrids for stretchable electronics. ACS Appl Mater Interf 13(49):59243–59251. https://doi.org/10.1021/acsami.1c17526
Jiang H, Duan L, Ren X et al (2019) Hydrophobic association hydrogels with excellent mechanical and self-healing properties. Eur Polym J 112:660–669. https://doi.org/10.1016/j.eurpolymj.2018.10.031
Zhou Y, Zhao S, Zhang C et al (2018) Photopolymerized maleilated chitosan/thiol-terminated poly (vinyl alcohol) hydrogels as potential tissue engineering scaffolds. Carbohydr Polym 184:383–389. https://doi.org/10.1016/j.carbpol.2018.01.009
Brown TE, Carberry BJ, Worrell BT et al (2018) Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials 178:496–503. https://doi.org/10.1016/j.biomaterials.2018.03.060
Zhou Y, Liang K, Zhao S et al (2018) Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int J Biol Macromol 108:383–390. https://doi.org/10.1016/j.ijbiomac.2017.12.032
Zhong Y, Wang J, Yuan Z et al (2019) A mussel-inspired carboxymethyl cellulose hydrogel with enhanced adhesiveness through enzymatic crosslinking. Colloids Surf B Biointerf 179:462–469. https://doi.org/10.1016/j.colsurfb.2019.03.044
Kim SH, Kim K, Kim BS et al (2020) Fabrication of polyphenol-incorporated anti-inflammatory hydrogel via high-affinity enzymatic crosslinking for wet tissue adhesion. Biomaterials 242:119905. https://doi.org/10.1016/j.biomaterials.2020.119905
Wei Q, Duan J, Ma G et al (2019) Enzymatic crosslinking to fabricate antioxidant peptide-based supramolecular hydrogel for improving cutaneous wound healing. J Mater Chem B 7(13):2220–2225. https://doi.org/10.1039/c8tb03147a
Wang H, Zhu D, Paul A et al (2017) Covalently adaptable elastin-like protein−hyaluronic acid (ELP−HA) hybrid hydrogels with secondary thermoresponsive crosslinking for injectable stem cell delivery. Adv Funct Mater 27(28):1605609. https://doi.org/10.1002/adfm.201605609
Shin JY, Yeo YH, Jeong JE et al (2020) Dual-crosslinked methylcellulose hydrogels for 3D bioprinting applications. Carbohydr Polym 238:116192. https://doi.org/10.1016/j.carbpol.2020.116192
Wang Y, Ma M, Wang J et al (2018) Development of a photo-crosslinking, biodegradable GelMA/PEGDA hydrogel for guided bone regeneration materials. Materials 11(8):1345. https://doi.org/10.3390/ma11081345
Kim HH, Kim JW, Choi J et al (2018) Characterization of silk hydrogel formed with hydrolyzed silk fibroin-methacrylate via photopolymerization. Polymer 153:232–240. https://doi.org/10.1016/j.polymer.2018.08.019
Spearman BS, Agrawal NK, Rubiano A et al (2020) Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J Biomed Mater Res A 108(2):279–291. https://doi.org/10.1002/jbm.a.36814
Xiao W, Qu X, Li J et al (2019) Synthesis and characterization of cell-laden double-network hydrogels based on silk fibroin and methacrylated hyaluronic acid. Eur Polym J 118:382–392. https://doi.org/10.1016/j.eurpolymj.2019.05.040
Teixeira LS, Feijen J, van Blitterswijk CA et al (2012) Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials 33(5):1281–1290. https://doi.org/10.1016/j.biomaterials.2011.10.067
Ranga A, Lutolf MP, Hilborn J et al (2016) Hyaluronic acid hydrogels formed in situ by transglutaminase-catalyzed reaction. Biomacromolecules 17(5):1553–1560. https://doi.org/10.1021/acs.biomac.5b01587
Parada GA, Yuk H, Liu X et al (2017) Impermeable robust hydrogels via hybrid lamination. Adv Healthc Mater 6(19):1700520. https://doi.org/10.1002/adhm.201700520
Hennink WE, van Nostrum CF (2012) Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev 64:223–236. https://doi.org/10.1016/j.addr.2012.09.009
Deng Z, Wang H, Ma PX et al (2020) Self-healing conductive hydrogels: preparation, properties and applications. Nanoscale 12(3):1224–1246. https://doi.org/10.1039/c9nr09283h
Mauri E, Negri A, Rebellato E et al (2018) Hydrogel-nanoparticles composite system for controlled drug delivery. Gels 4(3):74. https://doi.org/10.3390/gels4030074
Onaciu A, Munteanu RA, Moldovan AI et al (2019) Hydrogels based drug delivery synthesis, characterization and administration. Pharmaceutics 11(9):432. https://doi.org/10.3390/pharmaceutics11090432
Wang K, Hao Y, Wang Y et al (2019) Functional hydrogels and their application in drug delivery, biosensors, and tissue engineering. Int J Polym Sci 2019:1–14. https://doi.org/10.1155/2019/3160732
Han L, Yan L, Wang M et al (2018) Transparent, adhesive, and conductive hydrogel for soft bioelectronics based on light-transmitting polydopamine-doped polypyrrole nanofibrils. Chem Mater 30(16):5561–5572. https://doi.org/10.1021/acs.chemmater.8b01446
Wang X, Sun X, Gan D et al (2022) Bioadhesive and conductive hydrogel-integrated brain-machine interfaces for conformal and immune-evasive contact with brain tissue. Matter 5(4):1204–1223. https://doi.org/10.1016/j.matt.2022.01.012
Rothemund P, Kellaris N, Mitchell SK et al (2021) HASEL artificial muscles for a new generation of lifelike robots-recent progress and future opportunities. Adv Mater 33(19):e2003375. https://doi.org/10.1002/adma.202003375
Ha JH, Shin HH, Choi HW et al (2020) Electro-responsive hydrogel-based microfluidic actuator platform for photothermal therapy. Lab Chip 20(18):3354–3364. https://doi.org/10.1039/d0lc00458h
Song X, Wang C, Chen J et al (2021) Unraveling the synergistic coupling mechanism of Li+ transport in an “Ionogel-in-Ceramic” hybrid solid electrolyte for rechargeable lithium metal battery. Adv Funct Mater 32(10):2108706. https://doi.org/10.1002/adfm.202108706
Cheng FM, Chen HX, Li HD (2020) Recent advances in tough and self-healing nanocomposite hydrogels for shape morphing and soft actuators. Eur Polym J 124:109448. https://doi.org/10.1016/j.eurpolymj.2019.109448
Fantino E, Chiappone A, Roppolo I et al (2016) 3D printing of conductive complex structures with in situ generation of silver nanoparticles. Adv Mater 28(19):3712–3717. https://doi.org/10.1002/adma.201505109
Baei P, Jalili-Firoozinezhad S, Rajabi-Zeleti S et al (2016) Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Mater Sci Eng C Mater Biol Appl 63:131–141. https://doi.org/10.1016/j.msec.2016.02.056
Navaei A, Saini H, Christenson W et al (2016) Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater 41:133–146. https://doi.org/10.1016/j.actbio.2016.05.027
Walker BW, Lara RP, Mogadam E et al (2019) Rational design of microfabricated electroconductive hydrogels for biomedical applications. Prog Polym Sci 92:135–157. https://doi.org/10.1016/j.progpolymsci.2019.02.007
Liang Y, Zhao X, Hu T et al (2019) Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J Colloid Interf Sci 556:514–528. https://doi.org/10.1016/j.jcis.2019.08.083
Liang Y, Zhao X, Hu T et al (2019) Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small 15(12):e1900046. https://doi.org/10.1002/smll.201900046
Zhou Y, Fei X, Tian J et al (2022) A ionic liquid enhanced conductive hydrogel for strain sensing applications. J Colloid Interf Sci 606(Pt 1):192–203. https://doi.org/10.1016/j.jcis.2021.07.158
Dong R, Ma PX, Guo B (2020) Conductive biomaterials for muscle tissue engineering. Biomaterials 229:119584. https://doi.org/10.1016/j.biomaterials.2019.119584
Chakraborty P, Guterman T, Adadi N et al (2019) A self-healing, all-organic, conducting, composite peptide hydrogel as pressure sensor and electrogenic cell soft substrate. ACS Nano 13(1):163–175. https://doi.org/10.1021/acsnano.8b05067
Wang W, Narain R, Zeng H (2018) Rational design of self-healing tough hydrogels: a mini review. Front Chem 6:497. https://doi.org/10.3389/fchem.2018.00497
Guo Q, Chen J, Wang J et al (2020) Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale 12(3):1307–1324. https://doi.org/10.1039/c9nr09780e
Zhang G, Yang Y, Chen Y et al (2018) A quadruple-hydrogen-bonded supramolecular binder for high-performance silicon anodes in lithium-ion batteries. Small 14(29):e1801189. https://doi.org/10.1002/smll.201801189
Yi H, Lan T, Yang Y et al (2018) Aqueous-processable polymer binder with strong mechanical and polysulfide-trapping properties for high performance of lithium–sulfur batteries. J Mater Chem A 6(38):18660–18668. https://doi.org/10.1039/c8ta07194b
Huang W, Wang Y, Huang Z et al (2018) On-demand dissolvable self-healing hydrogel based on carboxymethyl chitosan and cellulose nanocrystal for deep partial thickness burn wound healing. ACS Appl Mater Interf 10(48):41076–41088. https://doi.org/10.1021/acsami.8b14526
Devaki SJ, Narayanan RK, Sarojam S (2014) Electrically conducting silver nanoparticle–polyacrylic acid hydrogel by in situ reduction and polymerization approach. Mater Lett 116:135–138. https://doi.org/10.1016/j.matlet.2013.10.110
Hyun DC, Park M, Park C et al (2011) Ordered zigzag stripes of polymer gel/metal nanoparticle composites for highly stretchable conductive electrodes. Adv Mater 23(26):2946–2950. https://doi.org/10.1002/adma.201100639
Han L, Liu K, Wang M et al (2018) Mussel-inspired adhesive and conductive hydrogel with long-lasting moisture and extreme temperature tolerance. Adv Funct Mater 28(3):1704195. https://doi.org/10.1002/adfm.201704195
Mottet L, Le Cornec D, Noel JM et al (2018) A conductive hydrogel based on alginate and carbon nanotubes for probing microbial electroactivity. Soft Matter 14(8):1434–1441. https://doi.org/10.1039/c7sm01929g
Lin X, Li F, Bing Y et al (2021) Biocompatible multifunctional e-skins with excellent self-healing ability enabled by clean and scalable fabrication. Nanomicro Lett 13(1):200. https://doi.org/10.1007/s40820-021-00701-8
Ilami M, Bagheri H, Ahmed R et al (2021) Materials, actuators, and sensors for soft bioinspired robots. Adv Mater 33(19):e2003139. https://doi.org/10.1002/adma.202003139
Lu B, Yuk H, Lin S et al (2019) Pure PEDOT:PSS hydrogels. Nat Commun 10(1):1043. https://doi.org/10.1038/s41467-019-09003-5
Yang C, Suo Z (2018) Hydrogel ionotronics. Nat Rev Mater 3(6):125–142. https://doi.org/10.1038/s41578-018-0018-7
Bai Y, Chen B, Xiang F et al (2014) Transparent hydrogel with enhanced water retention capacity by introducing highly hydratable salt. Appl Phys Lett 105:151903. https://doi.org/10.1063/1.4898189
Wang H, Zhu B, Jiang W et al (2014) A mechanically and electrically self-healing supercapacitor. Adv Mater 26(22):3638–3643. https://doi.org/10.1002/adma.201305682
Huang Y, Zhong M, Shi F et al (2017) An intrinsically stretchable and compressible supercapacitor containing a polyacrylamide hydrogel electrolyte. Angew Chem Int Ed Engl 56(31):9141–9145. https://doi.org/10.1002/anie.201705212
Zhao F, Bae J, Zhou X et al (2018) Nanostructured functional hydrogels as an emerging platform for advanced energy technologies. Adv Mater 30(48):e1801796. https://doi.org/10.1002/adma.201801796
Chen B, Chen Q, Xiao S et al (2021) Giant negative thermopower of ionic hydrogel by synergistic coordination and hydration interactions. Sci Adv 7(48):eabi7233. https://doi.org/10.1126/sciadv.abi7233
Huang Y, Li H, Wang Z et al (2016) Nanostructured polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 22:422–438. https://doi.org/10.1016/j.nanoen.2016.02.047
Huo Z, Tao L, Wang S et al (2015) A novel polysulfide hydrogel electrolyte based on low molecular mass organogelator for quasi-solid-state quantum dot-sensitized solar cells. J Power Sources 284:582–587. https://doi.org/10.1016/j.jpowsour.2015.03.049
Kellaris N, Gopaluni Venkata V, Smith Garrett M et al (2018) Peano-HASEL actuators: muscle-mimetic, electrohydraulic transducers that linearly contract on activation. Sci Robot 3(14):eaar3276. https://doi.org/10.1126/scirobotics.aar3276
Sun JY, Keplinger C, Whitesides GM et al (2014) Ionic skin. Adv Mater 26(45):7608–7614. https://doi.org/10.1002/adma.201403441
Li T, Li G, Liang Y et al (2017) Fast-moving soft electronic fish. Sci Adv 3(4):e1602045. https://doi.org/10.1126/sciadv.1602045
Yang H, Li C, Yang M et al (2019) Printing hydrogels and elastomers in arbitrary sequence with strong adhesion. Adv Funct Mater 29(27):1901721. https://doi.org/10.1002/adfm.201901721
Lei Z, Wang Q, Wu P (2017) A multifunctional skin-like sensor based on a 3D printed thermo-responsive hydrogel. Mater Horiz 4(4):694–700. https://doi.org/10.1039/c7mh00262a
Haghiashtiani G, Habtour E, Park SH et al (2018) 3D printed electrically-driven soft actuators. Extreme Mech Lett 21:1–8. https://doi.org/10.1016/j.eml.2018.02.002
Cheng FM, Chen HX, Li HD (2021) Recent progress on hydrogel actuators. J Mater Chem B 9(7):1762–1780. https://doi.org/10.1039/d0tb02524k
Jiang T, Munguia-Lopez JG, Flores-Torres S et al (2017) Directing the self-assembly of tumour spheroids by bioprinting cellular heterogeneous models within alginate/gelatin hydrogels. Sci Rep 7(1):4575. https://doi.org/10.1038/s41598-017-04691-9
Jiang T, Munguia-Lopez J, Flores-Torres S et al (2018) Bioprintable alginate/gelatin hydrogel 3D in vitro model systems induce cell spheroid formation. J Vis Exp 137:e57826. https://doi.org/10.3791/57826
Jiang T, Munguia-Lopez JG, Gu K et al (2019) Engineering bioprintable alginate/gelatin composite hydrogels with tunable mechanical and cell adhesive properties to modulate tumor spheroid growth kinetics. Biofabrication 12(1):015024. https://doi.org/10.1088/1758-5090/ab3a5c
Lin Z, Jiang T, Kinsella JM et al (2021) Assessing roughness of extrusion printed soft materials using a semi-quantitative method. Mater Lett 303:130480. https://doi.org/10.1016/j.matlet.2021.130480
Acome E, Mitchell SK, Morrissey TG et al (2018) Hydraulically amplified self-healing electrostatic actuators with muscle-like performance. Science 359(6371):61–65. https://doi.org/10.1126/science.aao6139
Mitchell SK, Wang X, Acome E et al (2019) An easy-to-implement toolkit to create versatile and high-performance HASEL actuators for untethered soft robots. Adv Sci 6(14):1900178. https://doi.org/10.1002/advs.201900178
Tian K, Bae J, Bakarich SE et al (2017) 3D printing of transparent and conductive heterogeneous hydrogel-elastomer systems. Adv Mater 29(10):1604827. https://doi.org/10.1002/adma.201604827
Yuk H, Lu B, Lin S et al (2020) 3D printing of conducting polymers. Nat Commun 11(1):1604. https://doi.org/10.1038/s41467-020-15316-7
Zhou LY, Fu J, He Y (2020) A review of 3D printing technologies for soft polymer materials. Adv Funct Mater 30(28):2000187. https://doi.org/10.1002/adfm.202000187
Handral HK, Natu VP, Cao T et al (2022) Emerging trends and prospects of electroconductive bioinks for cell-laden and functional 3D bioprinting. Bio-Des Manuf 5(2):396–411. https://doi.org/10.1007/s42242-021-00169-w
Jiang T, Munguia-Lopez JG, Flores-Torres S et al (2019) Extrusion bioprinting of soft materials: an emerging technique for biological model fabrication. Appl Phys Rev 6(1):011310. https://doi.org/10.1063/1.5059393
Ying GL, Jiang N, Maharjan S et al (2018) Aqueous two-phase emulsion bioink-enabled 3D bioprinting of porous hydrogels. Adv Mater 30(50):e1805460. https://doi.org/10.1002/adma.201805460
Truby RL, Wehner M, Grosskopf AK et al (2018) Soft somatosensitive actuators via embedded 3D printing. Adv Mater 30(15):e1706383. https://doi.org/10.1002/adma.201706383
Shao L, Gao Q, Xie C et al (2020) Synchronous 3D bioprinting of large-scale cell-laden constructs with nutrient networks. Adv Healthc Mater 9(15):e1901142. https://doi.org/10.1002/adhm.201901142
Shao L, Gao Q, Xie C et al (2020) Sacrificial microgel-laden bioink-enabled 3D bioprinting of mesoscale pore networks. Bio-Des Manuf 3(1):30–39. https://doi.org/10.1007/s42242-020-00062-y
Dong Z, Vuckovac M, Cui W et al (2021) 3D printing of superhydrophobic objects with bulk nanostructure. Adv Mater 33(45):e2106068. https://doi.org/10.1002/adma.202106068
Xue B, Sheng H, Li Y et al (2021) Stretchable and self-healable hydrogel artificial skin. Nat Sci Rev (Early Access). https://doi.org/10.1093/nsr/nwab147
Naficy S, Razal JM, Spinks GM et al (2012) Electrically conductive, tough hydrogels with pH sensitivity. Chem Mater 24(17):3425–3433. https://doi.org/10.1021/cm301666w
Liang X, Chen G, Lin S et al (2022) Bioinspired 2D isotropically fatigue-resistant hydrogels. Adv Mater 34(8):e2107106. https://doi.org/10.1002/adma.202107106
Guo G, Wu Q, Liu F et al (2021) Solvent-cast-assisted printing of biomimetic morphing hydrogel structures with solvent evaporation-induced swelling mismatch. Adv Funct Mater 32(2):2108548. https://doi.org/10.1002/adfm.202108548
Yuk H, Zhang T, Parada GA et al (2016) Skin-inspired hydrogel-elastomer hybrids with robust interfaces and functional microstructures. Nat Commun 7(1):12028. https://doi.org/10.1038/ncomms12028
Pissis P, Kyritsis A (1997) Electrical conductivity studies in hydrogels. Solid State Ion 97(1–4):105–113. https://doi.org/10.1016/s0167-2738(97)00074-x
Kaklamani G, Kazaryan D, Bowen J et al (2018) On the electrical conductivity of alginate hydrogels. Regen Biomater 5(5):293–301. https://doi.org/10.1093/rb/rby019
Chandra H, Allen SW, Oberloier SW et al (2017) Open-source automated mapping four-point probe. Materials 10(2):110. https://doi.org/10.3390/ma10020110
Zhao X, Chen F, Li Y et al (2018) Bioinspired ultra-stretchable and anti-freezing conductive hydrogel fibers with ordered and reversible polymer chain alignment. Nat Commun 9(1):3579. https://doi.org/10.1038/s41467-018-05904-z
Niiyama R, Rus D, Kim S (2014) Pouch motors: printable/inflatable soft actuators for robotics. In: IEEE international conference on robotics and automation, pp 6332–6337. https://doi.org/10.1109/ICRA.2014.6907793
Sanan S, Lynn PS, Griffith ST (2014) Pneumatic torsional actuators for inflatable robots. J Mech Robot 6(3):031003. https://doi.org/10.1115/1.4026629
Li W, Gao F, Wang X et al (2016) Strong and robust polyaniline-based supramolecular hydrogels for flexible supercapacitors. Angew Chem Int Ed Engl 55(32):9196–9201. https://doi.org/10.1002/anie.201603417
Li W, Lu H, Zhang N et al (2017) Enhancing the properties of conductive polymer hydrogels by freeze-thaw cycles for high-performance flexible supercapacitors. ACS Appl Mater Interf 9(23):20142–20149. https://doi.org/10.1021/acsami.7b05963
Chen Q, Lu H, Chen F et al (2018) Supramolecular hydrogels for high-voltage and neutral-ph flexible supercapacitors. ACS Appl Energy Mater 1(8):4261–4268. https://doi.org/10.1021/acsaem.8b00891
Lu H, Li Y, Chen Q et al (2019) Semicrystalline conductive hydrogels for high-energy and stable flexible supercapacitors. ACS Appl Energy Mater 2(11):8163–8172. https://doi.org/10.1021/acsaem.9b01629
Chen F, Chen Q, Song Q et al (2019) Strong and stretchable polypyrrole hydrogels with biphase microstructure as electrodes for substrate-free stretchable supercapacitors. Appl Mater Interf 6(11):1900133. https://doi.org/10.1002/admi.201900133
Pu X, Liu M, Chen X et al (2017) Ultrastretchable, transparent triboelectric nanogenerator as electronic skin for biomechanical energy harvesting and tactile sensing. Sci Adv 3(5):e1700015. https://doi.org/10.1126/sciadv.1700015
Zhou Y, Hou Y, Li Q et al (2017) Biocompatible and flexible hydrogel diode-based mechanical energy harvesting. Adv Mater Technol 2(9):1700118. https://doi.org/10.1002/admt.201700118
Parida K, Kumar V, Wang JX et al (2017) Highly transparent, stretchable, and self-healing ionic-skin triboelectric nanogenerators for energy harvesting and touch applications. Adv Mater 29(37):1702181. https://doi.org/10.1002/adma.201702181
Zhou D, Chen F, Handschuh-Wang S et al (2019) Biomimetic extreme-temperature- and environment-adaptable hydrogels. ChemPhysChem 20(17):2139–2154. https://doi.org/10.1002/cphc.201900545
Zhang S, Wang F, Peng H et al (2018) Flexible highly sensitive pressure sensor based on ionic liquid gel film. ACS Omega 3(3):3014–3021. https://doi.org/10.1021/acsomega.7b01575
Ren J, Liu Y, Wang Z et al (2021) An anti-swellable hydrogel strain sensor for underwater motion detection. Adv Funct Mater 32(13):2107404. https://doi.org/10.1002/adfm.202107404
Sarwar MS, Dobashi Y, Preston C et al (2017) Bend, stretch, and touch: locating a finger on an actively deformed transparent sensor array. Sci Adv 3(3):e1602200. https://doi.org/10.1126/sciadv.1602200
Li XH, Liu C, Feng SP et al (2019) Broadband light management with thermochromic hydrogel microparticles for smart windows. Joule 3(1):290–302. https://doi.org/10.1016/j.joule.2018.10.019
Choe A, Yeom J, Shanker R et al (2018) Stretchable and wearable colorimetric patches based on thermoresponsive plasmonic microgels embedded in a hydrogel film. NPG Asia Mater 10(9):912–922. https://doi.org/10.1038/s41427-018-0086-6
Bai N, Wang L, Wang Q et al (2020) Graded intrafillable architecture-based iontronic pressure sensor with ultra-broad-range high sensitivity. Nat Commun 11(1):209. https://doi.org/10.1038/s41467-019-14054-9
Cheng S, Narang YS, Yang C et al (2019) Stick-on large-strain sensors for soft robots. Adv Mater Interf 6(20):1900985. https://doi.org/10.1002/admi.201900985
Lin G, Si M, Wang L et al (2022) Dual-channel flexible strain sensors based on mechanofluorescent and conductive hydrogel laminates. Adv Opt Mater 10(5):2102306. https://doi.org/10.1002/adom.202102306
Li Y, Hu CX, Lan J et al (2020) Hydrogel-based temperature sensor with water retention, frost resistance and remoldability. Polymer 186:122027. https://doi.org/10.1016/j.polymer.2019.122027
Liu Z, Wang Y, Ren Y et al (2020) Poly(ionic liquid) hydrogel-based anti-freezing ionic skin for a soft robotic gripper. Maters Horiz 7(3):919–927. https://doi.org/10.1039/c9mh01688k
Yang CH, Chen B, Lu JJ et al (2015) Ionic cable. Extreme Mech Lett 3:59–65. https://doi.org/10.1016/j.eml.2015.03.001
Liu J, McKeon L, Garcia J et al (2022) Additive manufacturing of Ti3C2-MXene-functionalized conductive polymer hydrogels for electromagnetic-interference shielding. Adv Mater 34(5):e2106253. https://doi.org/10.1002/adma.202106253
Yeon SY, Yun J, Yoon SH et al (2018) A miniaturized solid salt reverse electrodialysis battery: a durable and fully ionic power source. Chem Sci 9(42):8071–8076. https://doi.org/10.1039/c8sc02954g
Lim SM, Yoo H, Oh MA et al (2019) Ion-to-ion amplification through an open-junction ionic diode. Proc Natl Acad Sci USA 116(28):13807–13815. https://doi.org/10.1073/pnas.1903900116
Lin S, Yuk H, Zhang T et al (2016) Stretchable hydrogel electronics and devices. Adv Mater 28(22):4497–4505. https://doi.org/10.1002/adma.201504152
Lee HR, Woo J, Han SH et al (2019) A stretchable ionic diode from copolyelectrolyte hydrogels with methacrylated polysaccharides. Adv Funct Mater 29(4):1806909. https://doi.org/10.1002/adfm.201806909
Bai R, Yang J, Morelle XP et al (2018) Fatigue fracture of self-recovery hydrogels. ACS Macro Lett 7(3):312–317. https://doi.org/10.1021/acsmacrolett.8b00045
Huang S, Liu Y, Zhao Y et al (2018) Flexible electronics: stretchable electrodes and their future. Adv Funct Mater 29(6):1805924. https://doi.org/10.1002/adfm.201805924
Vazquez-Gonzalez M, Willner I (2020) Stimuli-responsive biomolecule-based hydrogels and their applications. Angew Chem Int Ed Engl 59(36):15342–15377. https://doi.org/10.1002/anie.201907670
Wang J, Wang Z, Yu J et al (2020) Glucose-responsive insulin and delivery systems: innovation and translation. Adv Mater 32(13):e1902004. https://doi.org/10.1002/adma.201902004
Thakor J, Ahadian S, Niakan A et al (2020) Engineered hydrogels for brain tumor culture and therapy. Bio-Des Manuf 3(3):203–226. https://doi.org/10.1007/s42242-020-00084-6
Qu J, Zhao X, Ma PX et al (2018) Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater 72:55–69. https://doi.org/10.1016/j.actbio.2018.03.018
di Luca M, Vittorio O, Cirillo G et al (2018) Electro-responsive graphene oxide hydrogels for skin bandages: the outcome of gelatin and trypsin immobilization. Int J Pharm 546(1–2):50–60. https://doi.org/10.1016/j.ijpharm.2018.05.027
Fan Z, Cheng P, Zhang D et al (2020) Progress on stimulus responsive smart hydrogels based on natural polymers. Mater Rep 34(21):21012–21025 (in Chinese). https://doi.org/10.11896/cldb.19080216
Zhao F, Fan S, Ghate D et al (2022) A hydrogel ionic circuit based high-intensity iontophoresis device for intraocular macromolecule and nanoparticle delivery. Adv Mater 34(5):e2107315. https://doi.org/10.1002/adma.202107315
Bariya M, Nyein HYY, Javey A (2018) Wearable sweat sensors. Nat Electron 1(3):160–171. https://doi.org/10.1038/s41928-018-0043-y
Heikenfeld J, Jajack A, Feldman B et al (2019) Accessing analytes in biofluids for peripheral biochemical monitoring. Nat Biotechnol 37(4):407–419. https://doi.org/10.1038/s41587-019-0040-3
Kim J, Campbell AS, de Avila BE et al (2019) Wearable biosensors for healthcare monitoring. Nat Biotechnol 37(4):389–406. https://doi.org/10.1038/s41587-019-0045-y
Zhao C, Li X, Wu Q et al (2021) A thread-based wearable sweat nanobiosensor. Biosens Bioelectron 188:113270. https://doi.org/10.1016/j.bios.2021.113270
Banerjee H, Suhail M, Ren H (2018) Hydrogel actuators and sensors for biomedical soft robots: brief overview with impending challenges. Biomimetics 3(3):15. https://doi.org/10.3390/biomimetics3030015
Liu X, Liu J, Lin S et al (2020) Hydrogel machines. Mater Today 36:102–124. https://doi.org/10.1016/j.mattod.2019.12.026
Liu J, Zhang X, Liu Y et al (2020) Intrinsically stretchable electrode array enabled in vivo electrophysiological mapping of atrial fibrillation at cellular resolution. Proc Natl Acad Sci USA 117(26):14769–14778. https://doi.org/10.1073/pnas.2000207117
Zhang S, Ling H, Chen Y et al (2019) Hydrogel-enabled transfer-printing of conducting polymer films for soft organic bioelectronics. Adv Funct Mater 30(6):1906016. https://doi.org/10.1002/adfm.201906016
Wu J, Wu ZX, Xu HH et al (2019) An intrinsically stretchable humidity sensor based on anti-drying, self-healing and transparent organohydrogels. Mater Horiz 6(3):595–603. https://doi.org/10.1039/c8mh01160e
Elsherif M, Hassan MU, Yetisen AK et al (2018) Wearable contact lens biosensors for continuous glucose monitoring using smartphones. ACS Nano 12(6):5452–5462. https://doi.org/10.1021/acsnano.8b00829
Ying B, Wu Q, Li J et al (2020) An ambient-stable and stretchable ionic skin with multimodal sensation. Mater Horiz 7(2):477–488. https://doi.org/10.1039/c9mh00715f
Yu JC, Wang JQ, Zhang YQ et al (2020) Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat Biomed Eng 4(5):499–506. https://doi.org/10.1038/s41551-019-0508-y
Acknowledgements
This work was supported by the Research Project Funding of National University of Defense Technology of China (No. ZK19-33), the National Postdoctoral International Exchange Program Funding for Incoming Postdoctoral Students (Postdoctoral No. 48127), the Science and Technology Innovation Program of Hunan Province (No. 2020RC2036), and the National Natural Science Foundation of China (Nos. 52105039 and 52175069).
Author information
Authors and Affiliations
Contributions
YH investigated and summarized the literature and wrote the original draft. ZNL completed the analysis of statistical data and the production of the main pictures, and helped revise the paper. ZRL, TJ, and JZS helped revise the paper and gave some advice. YY gave some advice. ZRL, TJ, and JZS supervised the work and applied for funds. All authors have read and approved this manuscript for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This study does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hong, Y., Lin, Z., Luo, Z. et al. Development of conductive hydrogels: from design mechanisms to frontier applications. Bio-des. Manuf. 5, 729–756 (2022). https://doi.org/10.1007/s42242-022-00208-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-022-00208-0