Abstract
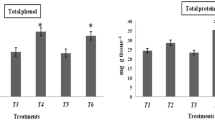
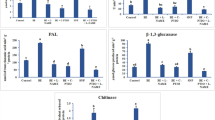
In the present investigation, emphasis was placed on the efficacy of lipopolysaccharide (LPS) extracted from the plant beneficial rhizobacterium Alcaligenes faecalis MSS8 for the induction of disease resistance by enhancing immune response in plants against phytopathogen Fusarium oxysporum f. sp. lycopersici (FOL). Characterization and proficiency of the rhizobacterial LPS for the induced defense response in plant against FOL was revealed by the gel activity staining of Peroxidase (PO) and Polyphenol oxidase (PPO) isoforms as compared to the untreated and only FOL treated plants. It has been observed that after 48 h of FOL challenge inoculation, in the LPS pretreated plant there was significant induction of PO and PPO isoforms. Furthermore, RT-PCR analysis for the study of relative gene expression of pathogenesis related (PR) proteins confirms the result of gel activity staining. It has been observed that expression level of LeChi9, LeGluB, LePR1a was significantly upregulated in LPS treated plants 24 h after FOL inoculation and maximum level was observed at 48 h as compared to the control and FOL treated plants. This clearly indicates the role of LPS extracted from A. faecalis as a potential biocontrol agent against fusarium wilt.





Similar content being viewed by others
References
Aimé S, Cordier C, Alabouvette C, Olivain C (2008) Comparative analysis of PR gene expression in tomato inoculated with virulent Fusarium oxysporum f. sp. lycopersici and the biocontrol strain F. oxysporum Fo47. Physiol Mol Plant Pathol 73(1):9–15
Gerber IB, Zeidler D, Durner J, Dubery IA (2004) Early perception responses of Nicotiana tabacum cells in response to lipopolysaccharides from Burkholderia cepacia. Planta 218:647–657
Haeffner-Cavaillon N, Caroff M, Cavaillon JM (1989) Interleukin-1 induction by lipopolysaccharides: structural requirements of the 3-deoxy-D-manno-2-octulosonic acid (KDO). Mol Immunol 26(5):485–494
Jetiyanon K, Plianbangchang P (2013) Lipopolysaccharide of Enterobacter strain RS83: a bacterial determinant for induction of early defensive enzymes in Lactuca sativa against soft rot disease. Biol Control 67:301–307
Jhala VM, Mandaliya VB, Thaker VS (2015) Simple and efficient protocol for RNA and DNA extraction from rice (Oryza sativa L.) for downstream applications. Int Res J Biol Sci 4(2):6247
Karkhanis YD, Zeltner JY, Jackson JJ, Carlo DJ (1977) A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem 85(2):595–601
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Mabrouk Y, Mejri S, Belhadj O (2016) Biochemical mechanisms of induced resistance by rhizobial lipopolysaccharide in pea against crenate broomrape. Braz J Bot 39(1):107–114
Marshall T (1984) Detection of protein in polyacrylamide gels using an improved silver stain. Anal Biochem 136(2):340–346
Nahar K, Kyndt T, De Vleesschauwer D, Höfte M, Gheysen G (2011) The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice. Plant Physiol 157:305–316
Nakano M, Niwa M, Nishimura N (2014) Development of a PCR-based method for monitoring the status of Alcaligenes species in the agricultural environment. Biocontrol Sci 19(1):23–31
Newman MA, Roepenack-Lahaye V, Parr A, Daniels MJ, Dow JM (2002) Prior exposure to lipopolysaccharide potentiates expression of plant defenses in response to bacteria. Plant J 29(4):487–495
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36
Reitz M, Rudolph K, Schröder I, Hoffmann-Hergarten S, Hallmann J, Sikora RA (2000) Lipopolysaccharides of rhizobium etli strain G12 act in potato roots as an inducing agent of systemic resistance to infection by the cyst nematode Globodera pallida. Appl Environ Microbiol 66:3515–3518
Roux M (2005) Phosphoprotein change in Arabidopsis thaliana in response to elicitation of lipopolysaccharides
Sagitov AO, El-Habbaa, GM, El-Fiki IA (2011) Studies on tomato wilt caused by Fusarium oxysporum f. sp. lycopersici in Kazachestan. 2: Effect of exogenous application of plant extracts and safe chemicals as resistance inducer treatments on the activity of the oxidative enzymes // Исследования Результаты (КазНАУ), г. Алматы, 2011. - №1(049), С 107-113.
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3(6):1101–1108
Sunayana MR, Reddy M (2015) Determination of keto-deoxy-d-manno-8-octanoic acid (KDO) from lipopolysaccharide of Escherichia coli. Bio-protocols 5(24). https://doi.org/10.21769/BioProtoc.1688.
Tsai CM, Frasch CE (1982) A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem 119(1):115–119
Acknowledgements
We are thankful to Department of Microbiology and Biotechnology, Gujarat University to encourage and help us with the required facility and work and GUJCOST MRP for financial support. Special thanks to Prof. Vrinda S. Thaker and her research students, UGC-CAS Department of Biosciences, Saurashtra University, Rajkot for her support and guidance during the RT- PCR analysis of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All Authors declares that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 438 kb)
Rights and permissions
About this article
Cite this article
Patel, S., Vrinda Thaker, Kuvad, R. et al. Role of lipopolysaccaride extracted from Alcaligenes faecalis as elicitor for the induction of plant defense against fusarium wilt. J Plant Pathol 102, 351–357 (2020). https://doi.org/10.1007/s42161-019-00425-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-019-00425-0