Abstract
Due to the large reserves, low cost, high security and high energy density, rechargeable multivalent batteries have attracted extensive research enthusiasm for a long time. Multivalent batteries are also supposed as the potential candidates to Li-ion batteries in portable electronic devices and large-scale energy storage units. Unfortunately, most commercial cathode materials in Li-ion batteries cannot be applied in multivalent batteries because of the intensive polarization problem of multivalent intercalated ions (Mg2+, Zn2+, Al3+). Choosing and synthesizing the appropriate cathode materials are the main issues in overcoming the intensive polarization problem. Vanadium-based materials often possess many kinds of oxidation states because of the mutable vanadium element, which can facilitate achieving local electroneutrality and relieve the polarization problem of multivalent ions. In this review, we summarize the researches about the vanadium-based cathode materials for multivalent batteries and highlight the intercalation mechanism of multivalent ions to vanadium-based materials. In addition, different kinds of optimizing strategies are extracted from the literatures. On the basis of our review, progresses and future challenges of vanadium-based cathode materials in rechargeable multivalent batteries are more explicit.
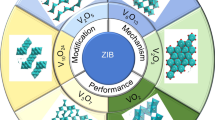
Graphical Abstract












Similar content being viewed by others
References
Dunn, B., Kamath, H., Tarascon, J.M.: Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011)
Andre, D., Kim, S.J., Lamp, P., et al.: Future generations of cathode materials: an automotive industry perspective. J. Mater. Chem. A 3, 6709–6732 (2015)
Erickson, E.M., Ghanty, C., Aurbach, D.: New horizons for conventional lithium ion battery technology. J. Phys. Chem. Lett. 5, 3313–3324 (2014)
Mai, L., Yan, M., Zhao, Y.: Track batteries degrading in real time. Nature 546, 469–470 (2017)
Nykvist, B., Nilsson, M.: Rapidly falling costs of battery packs for electric vehicles. Nat. Clim. Change 5, 329–332 (2015)
Sakti, A., Michalek, J.J., Fuchs, E.R.H., et al.: A techno-economic analysis and optimization of Li-ion batteries for light-duty passenger vehicle electrification. J. Power Sources 273, 966–980 (2015)
Whittingham, M.S.: Ultimate limits to intercalation reactions for lithium batteries. Chem. Rev. 114, 11414–11443 (2014)
Yang, Z., Zhang, J., Kintner-Meyer, M.C., et al.: Electrochemical energy storage for green grid. Chem. Rev. 111, 3577–3613 (2011)
Augustyn, V., Come, J., Lowe, M.A., et al.: High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 12, 518–522 (2013)
Bruce, P.G., Scrosati, B., Tarascon, J.M.: Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 47, 2930–2946 (2008)
Thackeray, M.M., Wolverton, C., Isaacs, E.D.: Electrical energy storage for transportation—approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 5, 7854–7863 (2012)
Ruth, M.F., Zinaman, O.R., Antkowiak, M., et al.: Nuclear-renewable hybrid energy systems: opportunities, interconnections, and needs. Energy Convers. Manag. 78, 684–694 (2014)
Chu, S., Majumdar, A.: Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012)
Heller, A.: Chemical engineering challenges and investment opportunities in sustainable energy. ChemSusChem 1, 651–652 (2008)
Pudukudy, M., Yaakob, Z., Mohammad, M., et al.: Renewable hydrogen economy in Asia—opportunities and challenges: an overview. Renew. Sustain. Energy Rev. 30, 743–757 (2014)
Shafiei, E., Davidsdottir, B., Leaver, J., et al.: Comparative analysis of hydrogen, biofuels and electricity transitional pathways to sustainable transport in a renewable-based energy system. Energy 83, 614–627 (2015)
Abruna, H.D., Kiya, Y., Henderson, J.C.: Batteries and electrochemical capacitors. Phys. Today 61, 43–47 (2008)
Crowther, O., West, A.C.: Effect of electrolyte composition on lithium dendrite growth. J. Electrochem. Soc. 155, A806–A811 (2008)
Zhamu, A., Chen, G., Liu, C., et al.: Reviving rechargeable lithium metal batteries: enabling next-generation high-energy and high-power cells. Energy Environ. Sci. 5, 5701–5707 (2012)
Crabtree, G.: The joint center for energy storage research: a new paradigm for battery research and development. In: Physics of Sustainable Energy III (PSE III): Using Energy Efficiently and Producing It Renewably, 3rd Physics of Sustainable Energy (PSE) Conference, Berkeley, March 2014. AIP Conference Proceedings, vol. 1652, pp. 112–128. American Institute of Physics, Melville (2015)
Berg, E.J., Villevieille, C., Streich, D., et al.: Rechargeable batteries: grasping for the limits of chemistry. J. Electrochem. Soc. 162, A2468–A2475 (2015)
Eroglu, D., Ha, S., Gallagher, K.G.: Fraction of the theoretical specific energy achieved on pack level for hypothetical battery chemistries. J. Power Sources 267, 14–19 (2014)
Gallagher, K.G., Goebel, S., Greszler, T., et al.: Quantifying the promise of lithium-air batteries for electric vehicles. Energy Environ. Sci. 7, 1555–1563 (2014)
Gallagher, K.G., Nelson, P.A., Dees, D.W.: Simplified calculation of the area specific impedance for battery design. J. Power Sources 196, 2289–2297 (2011)
Gallagher, K.G., Trask, S.E., Bauer, C., et al.: Optimizing areal capacities through understanding the limitations of lithium-ion electrodes. J. Electrochem. Soc. 163, A138–A149 (2016)
Lu, X., Kirby, B.W., Xu, W., et al.: Advanced intermediate-temperature Na–S battery. Energy Environ. Sci. 6, 299–306 (2012)
Lu, X., Lemmon, J.P., Kim, J.Y., et al.: High energy density Na–S/NiCl2 hybrid battery. J. Power Sources 224, 312–316 (2013)
Manwell, J.F., Mcgowan, J.G.: Lead acid battery storage model for hybrid energy systems. Sol. Energy 50, 399–405 (1993)
Abruna, H.D., Goodenough, J.B., Buchanan, M.V.: ANYL 28-Summary overview of basic research needs for electrical energy storage. In: Abstracts of Papers of the American Chemical Society, vol. 234, 28-ANYL. American Chemical Society, Washington, DC (2007)
Iwase, S., Minato, T.: Lead acid battery, has container, cover with exhaust port and projection disposed on peripheral portion of exhaust port that is formed in upper or side surface of cover. US Patent 2003049521-A1, 13 Mar 2003
Saiju, R., Heier, S.: Performance analysis of lead acid battery model for hybrid power system. In: 2008 IEEE/PES Transmission and Distribution Conference & Exposition, Chicago, April 2008. pp. 404-409. IEEE, Piscataway (2008)
Nogueira, C.A., Delmas, F.: New flowsheet for the recovery of cadmium, cobalt and nickel from spent Ni–Cd batteries by solvent extraction. Hydrometallurgy 52, 267–287 (1999)
Reddy, B.R., Priya, D.N., Rao, S.V., et al.: Solvent extraction and separation of Cd(II), Ni(II) and Co(II) from chloride leach liquors of spent Ni–Cd batteries using commercial organo-phosphorus extractants. Hydrometallurgy 77, 253–261 (2005)
Sun, B., Skyllas-Kazacos, M.: Modification of graphite electrode materials for vanadium redox flow battery application—I. Thermal treatment. Electrochim. Acta 37, 1253–1260 (1992)
Liu, Q.H., Grim, G.M., Papandrew, A.B., et al.: High performance vanadium redox flow batteries with optimized electrode configuration and membrane selection. J. Electrochem. Soc. 159, A1246–A1252 (2012)
Sun, B., Skyllas-Kazacos, M.: Chemical modification of graphite electrode materials for vanadium redox flow battery application. Part II. Acid treatments. Electrochim. Acta. 37, 2459–2465 (1992)
Jian, Z., Han, W., Liang, Y., et al.: Carbon-coated rhombohedral Li3V2(PO4)3 as both cathode and anode materials for lithium-ion batteries: electrochemical performance and lithium storage mechanism. J. Mater. Chem. A 2, 20231–20236 (2014)
Song, W., Ji, X., Pan, C., et al.: A Na3V2(PO4)3 cathode material for use in hybrid lithium ion batteries. Phys. Chem. Chem. Phys. 15, 14357–14363 (2013)
Song, W., Ji, X., Yao, Y., et al.: A promising Na3V2(PO4)3 cathode for use in the construction of high energy batteries. Phys. Chem. Chem. Phys. 16, 3055–3061 (2014)
Liu, J., Zhang, J.G., Yang, Z., et al.: Materials science and materials chemistry for large scale electrochemical energy storage: from transportation to electrical grid. Adv. Funct. Mater. 23, 929–946 (2013)
Plashnitsa, L.S., Kobayashi, E., Noguchi, Y., et al.: Performance of NASICON symmetric cell with ionic liquid electrolyte. J. Electrochem. Soc. 157, A536–A543 (2010)
Yao, Y., Mcdowell, M.T., Ryu, I., et al.: Interconnected silicon hollow nanospheres for lithium-ion battery anodes with long cycle life. Nano Lett. 11, 2949–2954 (2011)
Fergus, J.W.: Recent developments in cathode materials for lithium ion batteries. J. Power Sources 195, 939–954 (2010)
He, C., Wu, S., Zhao, N., et al.: Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material. ACS Nano 7, 4459–4469 (2013)
Canepa, P., Gautam, G.S., Hannah, D.C., et al.: Odyssey of multivalent cathode materials: open questions and future challenges. Chem. Rev. 117, 4287–4341 (2017)
Massé, R.C., Uchaker, E., Cao, G.: Beyond Li-ion: electrode materials for sodium- and magnesium-ion batteries. Sci. China Mater. 58, 715–766 (2015)
Levi, E., Gofer, Y., Aurbach, D.: On the way to rechargeable mg batteries: the challenge of new cathode materials. Chem. Mater. 22, 860–868 (2010)
Saha, P., Datta, M.K., Velikokhatnyi, O.I., et al.: Rechargeable magnesium battery: current status and key challenges for the future. Prog. Mater Sci. 66, 1–86 (2014)
Elia, G.A., Marquardt, K., Hoeppner, K., et al.: An overview and future perspectives of aluminum batteries. Adv. Mater. 28, 7564–7579 (2016)
Pan, H.L., Shao, Y.Y., Yan, P.F., et al.: Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 1, 16039 (2016)
Gershinsky, G., Yoo, H.D., Gofer, Y., et al.: Electrochemical and spectroscopic analysis of Mg2+ intercalation into thin film electrodes of layered oxides: V2O5 and MoO3. Langmuir 29, 10964–10972 (2013)
Aurbach, D., Weissman, I., Gofer, Y., et al.: Nonaqueous magnesium electrochemistry and its application in secondary batteries. Chem. Rec. 3, 61–73 (2003)
Brissot, C., Rosso, M., Chazalviel, J.N., et al.: Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 81–82, 925–929 (1999)
Shterenberg, I., Salama, M., Gofer, Y., et al.: The challenge of developing rechargeable magnesium batteries. MRS Bull. 39, 453–460 (2014)
Yoo, H.D., Shterenberg, I., Gofer, Y., et al.: Mg rechargeable batteries: an on-going challenge. Energy Environ. Sci. 6, 2265–2279 (2013)
Besenhard, J.O., Winter, M.: Advances in battery technology: rechargeable magnesium batteries and novel negative-electrode materials for lithium ion batteries. ChemPhysChem 3, 155–159 (2002)
Cheng, Y., Liu, T., Shao, Y., et al.: Electrochemically stable cathode current collectors for rechargeable magnesium batteries. J. Mater. Chem. A 2, 2473–2477 (2014)
Gregory, T.D., Hoffman, R.J., Winterton, R.C.: Nonaqueous electrochemistry of magnesium; applications to energy storage. J. Electrochem. Soc. 137, 775–780 (1990)
Aurbach, D., Lu, Z., Schechter, A., et al.: Prototype systems for rechargeable magnesium batteries. Nature 407, 724–727 (2000)
Gershinsky, G., Haik, O., Salitra, G., et al.: Ultra fast elemental synthesis of high yield copper Chevrel phase with high electrochemical performance. J. Solid State Chem. 188, 50–58 (2012)
Levi, E., Mitelman, A., Isnard, O., et al.: Phase diagram of Mg insertion into Chevrel phases, Mg x Mo6T8 (T = S, Se). 3. The crystal structure of triclinic Mg2Mo6Se8. Inorg. Chem. 47, 1975–1983 (2008)
Levi, M.D., Lancri, E., Levi, E., et al.: The effect of the anionic framework of Mo6X8 Chevrel Phase (X = S, Se) on the thermodynamics and the kinetics of the electrochemical insertion of Mg2+ ions. Solid State Ion. 176, 1695–1699 (2005)
Mitelman, A., Levi, E., Lancry, E., et al.: On the Mg trapping mechanism in electrodes comprising Chevrel phases. ECS Trans. 3, 109–115 (2007)
Mitelman, A., Levi, M.D., Lancry, E., et al.: New cathode materials for rechargeable Mg batteries: fast Mg ion transport and reversible copper extrusion in Cu y Mo6S8 compounds. Chem. Commun. 41, 4212–4214 (2007)
Suresh, G.S., Levi, M.D., Aurbach, D.: Effect of chalcogen substitution in mixed Mo6S8−nSe n (n = 0, 1, 2) Chevrel phases on the thermodynamics and kinetics of reversible Mg ions insertion. Electrochim. Acta 53, 3889–3896 (2008)
Tao, Z.L., Xu, L.N., Gou, X.L., et al.: TiS2 nanotubes as the cathode materials of Mg-ion batteries. Chem. Commun. 18, 2080–2081 (2004)
Arthur, T.S., Zhang, R., Ling, C., et al.: Understanding the electrochemical mechanism of K-αMnO2 for magnesium battery cathodes. ACS Appl. Mater. Interfaces 6, 7004–7008 (2014)
Huang, Z.D., Masese, T., Orikasa, Y., et al.: MgFePO4F as a feasible cathode material for magnesium batteries. J. Mater. Chem. A 2, 11578–11582 (2014)
Ichitsubo, T., Adachi, T., Yagi, S., et al.: Potential positive electrodes for high-voltage magnesium-ion batteries. J. Mater. Chem. 21, 11764–11772 (2011)
Kim, C., Phillips, P.J., Key, B., et al.: Direct observation of reversible magnesium ion intercalation into a spinel oxide host. Adv. Mater. 27, 3377–3384 (2015)
Ling, C., Zhang, R., Arthur, T.S., et al.: How general is the conversion reaction in Mg battery cathode: a case study of the magnesiation of α-MnO2. Chem. Mater. 27, 5799–5807 (2015)
Muldoon, J., Bucur, C.B., Gregory, T.: Quest for nonaqueous multivalent secondary batteries: magnesium and beyond. Chem. Rev. 114, 11683–11720 (2014)
Orikasa, Y., Masese, T., Koyama, Y., et al.: High energy density rechargeable magnesium battery using earth-abundant and non-toxic elements. Sci. Rep. 4, 5622 (2014)
Wang, R.Y., Wessells, C.D., Huggins, R.A., et al.: Highly reversible open framework nanoscale electrodes for divalent ion batteries. Nano Lett. 13, 5748–5752 (2013)
Zhang, R., Mizuno, F., Ling, C.: Fullerenes: non-transition metal clusters as rechargeable magnesium battery cathodes. Chem. Commun. 51, 1108–1111 (2015)
Zheng, Y., Nuli, Y., Chen, Q., et al.: Magnesium cobalt silicate materials for reversible magnesium ion storage. Electrochim. Acta 66, 75–81 (2012)
Aurbach, D., Gizbar, H., Schechter, A., et al.: Electrolyte solutions for rechargeable magnesium batteries based on organomagnesium chloroaluminate complexes. J. Electrochem. Soc. 149, A115–A121 (2002)
Xu, C., Li, B., Du, H., et al.: Energetic zinc ion chemistry: the rechargeable zinc ion battery. Angew. Chem. Int. Ed. 51, 933–935 (2012)
Mclarnon, F.R., Cairns, E.J.: The secondary alkaline zinc electrode. J. Electrochem. Soc. 138, 645–664 (1991)
Lee, B., Lee, H.R., Kim, H., et al.: Elucidating the intercalation mechanism of zinc ions into α-MnO2 for rechargeable zinc batteries. Chem. Commun. 51, 9265–9268 (2015)
Alfaruqi, M.H., Mathew, V., Gim, J., et al.: Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system. Chem. Mater. 27, 3609–3620 (2015)
Hertzberg, B.J., Huang, A., Hsieh, A., et al.: Effect of multiple cation electrolyte mixtures on rechargeable Zn–MnO2 alkaline battery. Chem. Mater. 28, 4536–4545 (2016)
Zhang, N., Cheng, F., Liu, J., et al.: Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 8, 405 (2017)
Zhang, L., Chen, L., Zhou, X., et al.: Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: the zinc/zinc hexacyanoferrate system. Adv. Energy. Mater. 5, 1400930 (2015)
Trócoli, R., La Mantia, F.: An aqueous zinc-ion battery based on copper hexacyanoferrate. ChemSusChem 8, 481–485 (2015)
Jayaprakash, N., Das, S.K., Archer, L.A.: The rechargeable aluminum-ion battery. Chem. Commun. 47, 12610–12612 (2011)
Li, Q., Bjerrum, N.J.: Aluminum as anode for energy storage and conversion: a review. J. Power Sources 110, 1–10 (2002)
Zafar, Z.A., Imtiaz, S., Razaq, R., et al.: Cathode materials for rechargeable aluminum batteries: current status and progress. J. Mater. Chem. A 5, 5646–5660 (2017)
He, Y.J., Peng, J.F., Chu, W., et al.: Black mesoporous anatase TiO2 nanoleaves: a high capacity and high rate anode for aqueous Al-ion batteries. J. Mater. Chem. A. 2, 1721–1731 (2014)
Lin, M.C., Gong, M., Lu, B., et al.: An ultrafast rechargeable aluminium-ion battery. Nature 520, 325–328 (2015)
Jung, S.C., Kang, Y.-J., Yoo, D.-J., et al.: Flexible few-layered graphene for the ultrafast rechargeable aluminum-ion battery. J. Phys. Chem. C 120, 13384–13389 (2016)
Chen, H., Guo, F., Liu, Y., et al.: A defect-free principle for advanced graphene cathode of aluminum-ion battery. Adv. Mater. 29, 1605958 (2017)
Takami, N., Koura, N.: Electrochemical behavior of FeS2 cathodes for aluminum secondary cells around 100 °C. J. Electrochem. Soc. 140, 928–932 (1993)
Wang, S., Yu, Z., Tu, J., et al.: A novel aluminum-ion battery: Al/AlCl3-[EMIm]Cl/Ni3S2@Graphene. Adv. Energy Mater. 6, 1600137 (2016)
Wang, S., Jiao, S., Wang, J., et al.: High-performance aluminum-ion battery with CuS@C microsphere composite cathode. ACS Nano 11, 469–477 (2017)
Donahue, F.M., Mancini, S.E., Simonsen, L.: Secondary aluminium-iron (III) chloride batteries with a low temperature molten salt electrolyte. J. Appl. Electrochem. 22, 230–234 (1992)
Zhao, Y., Han, C., Yang, J., et al.: Stable alkali metal ion intercalation compounds as optimized metal oxide nanowire cathodes for lithium batteries. Nano Lett. 15, 2180–2185 (2015)
An, Q., Wei, Q., Zhang, P., et al.: Three-dimensional interconnected vanadium pentoxide nanonetwork cathode for high-rate long-life lithium batteries. Small 11, 2654–2660 (2015)
An, Q., Zhang, P., Wei, Q., et al.: Top-down fabrication of three-dimensional porous V2O5 hierarchical microplates with tunable porosity for improved lithium battery performance. J. Mater. Chem. A. 2, 3297–3302 (2014)
Huie, M.M., Bock, D.C., Takeuchi, E.S., et al.: Cathode materials for magnesium and magnesium-ion based batteries. Coordin. Chem. Rev. 287, 15–27 (2015)
Liu, M., Rong, Z., Malik, R., et al.: Spinel compounds as multivalent battery cathodes: a systematic evaluation based on ab initio calculations. Energy Environ. Sci. 8, 964–974 (2015)
Novák, P., Imhof, R., Haas, O.: Magnesium insertion electrodes for rechargeable nonaqueous batteries—a competitive alternative to lithium? Electrochim. Acta 45, 351–367 (1999)
Novák, P., Scheifele, W., Joho, F., et al.: Electrochemical insertion of magnesium into hydrated vanadium bronzes. J. Electrochem. Soc. 142, 2544–2550 (1995)
Novák, P., Desilvestro, J.: Electrochemical insertion of magnesium in metal oxides and sulfides from aprotic electrolytes. J. Electrochem. Soc. 140, 140–144 (1993)
Le, D.B., Passerini, S., Coustier, F., et al.: Intercalation of polyvalent cations into V2O5 aerogels. Chem. Mater. 10, 682–684 (1998)
Wang, Z., Su, Q., Deng, H.: Single-layered V2O5 a promising cathode material for rechargeable Li and Mg ion batteries: an ab initio study. Phys. Chem. Chem. Phys. 15, 8705–8709 (2013)
Zhou, B., Shi, H., Cao, R., et al.: Theoretical study on the initial stage of a magnesium battery based on a V2O5 cathode. Phys. Chem. Chem. Phys. 16, 18578–18585 (2014)
Gautam, G.S., Canepa, P., Richards, W.D., et al.: Role of structural H2O in intercalation electrodes: the case of Mg in nanocrystalline xerogel-V2O5. Nano Lett. 16, 2426–2431 (2016)
Tepavcevic, S., Liu, Y., Zhou, D., et al.: Nanostructured layered cathode for rechargeable Mg-ion batteries. ACS Nano 9, 8194–8205 (2015)
Lee, S.H., Dileo, R., Marschilok, A.C., et al.: Sol gel based synthesis and electrochemistry of magnesium vanadium oxide: a promising cathode material for secondary magnesium ion batteries. ECS Electrochem. Lett. 3, A87–A90 (2014)
Imamura, D., Miyayama, M., Hibino, M., et al.: Mg intercalation properties into V2O5 gel/carbon composites under high-rate condition. J. Electrochem. Soc. 150, A753–A758 (2003)
Tang, P.E., Sakamoto, J.S., Baudrin, E., et al.: V2O5 aerogel as a versatile host for metal ions. J. Non-Cryst. Solids 350, 67–72 (2004)
Cheng, Y., Shao, Y., Raju, V., et al.: Molecular storage of Mg ions with vanadium oxide nanoclusters. Adv. Funct. Mater. 26, 3446–3453 (2016)
An, Q., Li, Y., Yoo, D.H., et al.: Graphene decorated vanadium oxide nanowire aerogel for long-cycle-life magnesium battery cathodes. Nano. Energy 18, 265–272 (2015)
Du, X., Huang, G., Qin, Y., et al.: Solvothermal synthesis of GO/V2O5 composites as a cathode material for rechargeable magnesium batteries. RSC Adv. 5, 76352–76355 (2015)
Park, S.H., Lee, Y.S., Sun, Y.K.: Synthesis and electrochemical properties of sulfur doped-Li x MnO2−yS y materials for lithium secondary batteries. Electrochem. Commun. 5, 124–128 (2003)
Inamoto, M., Kurihara, H., Yajima, T.: Vanadium pentoxide-based composite synthesized using microwave water plasma for cathode material in rechargeable magnesium batteries. Materials 6, 4514–4522 (2013)
Arthur, T., Kato, K., Germain, J., et al.: Amorphous V2O5–P2O5 as high-voltage cathodes for magnesium batteries. Chem. Commun. 51, 15657–15660 (2015)
Guerra, E.M., Ciuffi, K.J., Oliveira, H.P.: V2O5 xerogel-poly(ethylene oxide) hybrid material: synthesis, characterization, and electrochemical properties. J. Solid State Chem. 179, 3814–3823 (2006)
Chao, D., Xia, X., Liu, J., et al.: A V2O5/conductive-polymer core/shell nanobelt array on three- dimensional graphite foam: a high-rate, ultrastable, and freestanding cathode for lithium-ion batteries. Adv. Mater. 26, 5794–5800 (2014)
Huguenin, F., Torresi, R.M.: Investigation of the electrical and electrochemical properties of nanocomposites from V2O5, polypyrrole, and polyaniline. J. Phys. Chem. C 112, 2202–2209 (2008)
Reddy, C.V.S., Wei, J., Quan-Tao, Z., et al.: Cathodic performance of (V2O5 + PEG) nanobelts for Li ion rechargeable battery. J. Power Sources 166, 244–249 (2007)
Shao, L., Jeon, J.W., Lutkenhaus, J.: Porous polyaniline nanofiber/vanadium pentoxide layer-by-layer electrodes for energy storage. J. Mater. Chem. A 1, 7648–7656 (2013)
Perera, S.D., Archer, R.B., Damin, C.A., et al.: Controlling interlayer interactions in vanadium pentoxide-poly(ethylene oxide) nanocomposites for enhanced magnesium-ion charge transport and storage. J. Power Sources 343, 580–591 (2017)
Kulish, V.V., Manzhos, S.: Comparison of Li, Na, Mg and Al-ion insertion in vanadium pentoxides and vanadium dioxides. RSC Adv. 7, 18643–18649 (2017)
Niu, C., Meng, J., Han, C., et al.: VO2 nanowires assembled into hollow microspheres for high-rate and long-life lithium batteries. Nano Lett. 14, 2873–2878 (2014)
Luo, T., Liu, Y., Su, H., et al.: Nanostructured-VO2(B): a high-capacity magnesium-ion cathode and its electrochemical reaction mechanism. Electrochim. Acta 260, 805–813 (2018)
Won, J.M., Ko, Y.N., Lee, J.K., et al.: Superior electrochemical properties of rutile VO2-carbon composite microspheres as a promising anode material for lithium ion batteries. Electrochim. Acta 156, 179–187 (2015)
Pei, C., Xiong, F., Sheng, J., et al.: VO2 nanoflakes as the cathode material of hybrid magnesium–lithium-ion batteries with high energy density. ACS. Appl. Mater. Inter. 9, 17060–17066 (2017)
Jiao, L., Yuan, H., Si, Y., et al.: Electrochemical insertion of magnesium in open-ended vanadium oxide nanotubes. J. Power Sources 156, 673–676 (2006)
Kim, R.H., Kim, J.S., Kim, H.J., et al.: Highly reduced VO x nanotube cathode materials with ultra-high capacity for magnesium ion batteries. J. Mater. Chem. A. 2, 20636–20641 (2014)
Jiao, L., Yuan, H., Wang, Y., et al.: Mg intercalation properties into open-ended vanadium oxide nanotubes. Electrochem. Commun. 7, 431–436 (2005)
Patzke, G.R., Krumeich, F., Nesper, R.: Oxidic nanotubes and nanorods-anisotropic modules for a future nanotechnology. Angew. Chem. Int. Ed. 41, 2446–2461 (2002)
Novák, P., Scheifele, W., Haas, O.: Magnesium insertion batteries-an alternative to lithium? J. Power Sources 54, 479–482 (1995)
Rashad, M., Zhang, H., Asif, M., et al.: Low cost room temperature synthesis of NaV3O8.1.69H2O nanobelts for Mg batteries. ACS Appl. Mater. Interfaces 10, 4757–4766 (2018)
Cabello, M., Nacimiento, F., Alcántara, R., et al.: Nanobelts of beta-sodium vanadate as electrode for magnesium and dual magnesium-sodium batteries. J. Electrochem. Soc. 163, A2781–A2790 (2016)
Chen, B., Laverock, J., Newby, D., et al.: Electronic structure of β-Na x V2O5 (x ≈ 0.33) polycrystalline films: growth, spectroscopy, and theory. J. Phys. Chem. C 118, 1081–1094 (2014)
Delmas, C., Nadiri, A., Soubeyroux, J.L.: The nasicon-type titanium phosphates ATi2(PO4)3 (A = Li, Na) as electrode materials. Solid State Ion. 28–30((Part 1)), 419–423 (1988)
Ong, S.P., Chevrier, V.L., Hautier, G., et al.: Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials. Energy Environ. Sci. 4, 3680–3688 (2011)
Shi, J.J., Yin, G.Q., Jing, L.M., et al.: Lithium and sodium diffusion in solid electrolyte materials of AM2(PO4)3(A = Li, Na, M = Ti, Sn and Zr). Int. J. Mod. Phys. B 28, 1450176 (2014)
Kang, J., Chung, H., Doh, C., et al.: Integrated study of first principles calculations and experimental measurements for Li-ionic conductivity in Al-doped solid-state LiGe2(PO4)3 electrolyte. J. Power Sources 293, 11–16 (2015)
Goodenough, J.B., Hong, Y.P., Kafalas, J.A.: Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 11, 203–220 (1976)
Makino, K., Katayama, Y., Miura, T., et al.: Electrochemical insertion of magnesium to Mg0.5Ti2(PO4)3. J. Power Sources 99, 66–69 (2001)
Huang, Z.D., Masese, T., Orikasa, Y., et al.: Vanadium phosphate as a promising high-voltage magnesium ion (de)-intercalation cathode host. RSC Adv. 5, 8598–8603 (2015)
Kim, J., Yoo, J.K., Jung, Y.S., et al.: Li3V2(PO4)3/conducting polymer as a high power 4 V-class lithium battery electrode. Adv. Energy Mater. 3, 1004–1007 (2013)
Liu, H., Gao, P., Fang, J., et al.: Li3V2(PO4)3/graphene nanocomposites as cathode material for lithium ion batteries. Chem. Commun. 47, 9110–9112 (2011)
Muldoon, J., Bucur, C.B., Oliver, A.G., et al.: Electrolyte roadblocks to a magnesium rechargeable battery. Energy Environ. Sci. 5, 5941–5950 (2012)
Canepa, P., Gautam, G.S., Malik, R., et al.: Understanding the initial stages of reversible Mg deposition and stripping in inorganic nonaqueous electrolytes. Chem. Mater. 27, 3317–3325 (2015)
Canepa, P., Jayaraman, S., Cheng, L., et al.: Elucidating the structure of the magnesium aluminum chloride complex electrolyte for magnesium-ion batteries. Energy Environ. Sci. 8, 3718–3730 (2015)
Cabello, M., Alcántara, R., Nacimiento, F., et al.: Na3V2(PO4)3 as electrode material for rechargeable magnesium batteries: a case of sodium-magnesium hybrid battery. Electrochim. Acta 246, 908–913 (2017)
Li, Y., An, Q., Cheng, Y., et al.: A high-voltage rechargeable magnesium-sodium hybrid battery. Nano Energy 34, 188–194 (2017)
Zeng, J., Yang, Y., Lai, S., et al.: A promising high-voltage cathode material based on mesoporous Na3V2(PO4)3/C for rechargeable magnesium batteries. Chem-Eur. J. 23, 16898–16905 (2017)
Prosini, P.P., Lisi, M., Zane, D., et al.: Determination of the chemical diffusion coefficient of lithium in LiFePO4. Solid State Ion. 148, 45–51 (2002)
Rui, X.H., Ding, N., Liu, J., et al.: Analysis of the chemical diffusion coefficient of lithium ions in Li3V2(PO4)3 cathode material. Electrochim. Acta 55, 2384–2390 (2010)
Böckenfeld, N., Balducci, A.: Determination of sodium ion diffusion coefficients in sodium vanadium phosphate. J. Solid State Electrochem. 18, 959–964 (2014)
Kaveevivitchai, W., Jacobson, A.J.: High capacity rechargeable magnesium-ion batteries based on a microporous molybdenum–vanadium oxide cathode. Chem. Mater. 28, 4593–4601 (2016)
Miao, X., Chen, Z., Wang, N., et al.: Electrospun V2MoO8 as a cathode material for rechargeable batteries with Mg metal anode. Nano Energy 34, 26–35 (2017)
Cho, J.H., Aykol, M., Kim, S., et al.: Controlling the intercalation chemistry to design high-performance dual-salt hybrid rechargeable batteries. J. Am. Chem. Soc. 136, 16116–16119 (2014)
Gao, T., Han, F., Zhu, Y., et al.: Hybrid Mg2+/Li+ battery with long cycle life and high rate capability. Adv. Energy Mater. 5, 1401507 (2015)
Zhang, Z., Xu, H., Cui, Z., et al.: High energy density hybrid Mg2+/Li+ battery with superior ultra-low temperature performance. J. Mater. Chem. A 4, 2277–2285 (2016)
Minella, C.B., Gao, P., Zhao-Karger, Z., et al.: Interlayer-expanded vanadium oxychloride as an electrode material for magnesium-based batteries. ChemElectroChem 4, 738–745 (2017)
Hannah, D.C., Gautam, G.S., Canepa, P., et al.: Magnesium ion mobility in post-spinels accessible at ambient pressure. Chem. Commun. 53, 5171–5174 (2017)
Ling, C., Mizuno, F.: Phase stability of post-spinel compound AMn2O4 (A = Li, Na, or Mg) and its application as a rechargeable battery cathode. Chem. Mater. 25, 3062–3071 (2013)
Sun, X., Blanc, L., Nolis, G.M., et al.: NaV1.25Ti0.75O4: a potential post spinel cathode material for Mg batteries. Chem. Mater. 30, 121–128 (2018)
Yan, M., He, P., Chen, Y., et al.: Water-lubricated intercalation in V2O5·H2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv. Mater. 30, 1703725 (2018)
Senguttuvan, P., Han, S.D., Kim, S., et al.: A high power rechargeable nonaqueous multivalent Zn/V2O5 battery. Adv. Energy Mater. 6, 1600826 (2016)
Kundu, D., Adams, B.D., Ort, V.D., et al.: A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 1, 16119 (2016)
Baghbanzadeh, M., Carbone, L., Cozzoli, P.D., et al.: Microwave-assisted synthesis of colloidal inorganic nanocrystals. Angew. Chem. Int. Ed. 50, 11312–11359 (2011)
He, P., Zhang, G., Liao, X., et al.: Sodium ion stabilized vanadium oxide nanowire cathode for high-performance zinc-ion batteries. Adv. Energy. Mater 8, 1702463 (2018)
Xia, C., Guo, J., Lei, Y., et al.: Rechargeable aqueous zinc-ion battery based on porous framework zinc pyrovanadate intercalation cathode. Adv. Mater. 30, 1705580 (2018)
Alfaruqi, M.H., Mathew, V., Song, J., et al.: Electrochemical zinc intercalation in lithium vanadium oxide: a high-capacity zinc-ion battery cathode. Chem. Mater. 29, 1684–1694 (2017)
Tang, H., Xu, N., Pei, C., et al.: H2V3O8 nanowires as high-capacity cathode materials for magnesium-based battery. ACS. Appl. Mater. Interfaces 9, 28667–28673 (2017)
He, P., Quan, Y., Xu, X., et al.: High-performance aqueous zinc-ion battery based on layered H2V3O8 nanowire cathode. Small 13, 1702551 (2017)
Li, H., He, P., Wang, Y., et al.: High-surface vanadium oxides with large capacities for lithium-ion batteries: from hydrated aerogel to nanocrystalline VO2(B), V6O13 and V2O5. J. Mater. Chem. A 21, 10999–11009 (2011)
Sarkar, S., Bhowmik, A., Pan, J., et al.: Preparation, structure study and electrochemistry of layered H2V3O8 materials: high capacity lithium-ion battery cathode. J. Power Sources 329, 179–189 (2016)
Su, D., Wang, G.: Single-crystalline bilayered V2O5 nanobelts for high-capacity sodium-ion batteries. ACS Nano 7, 11218–11226 (2013)
Li, G., Yang, Z., Jiang, Y., et al.: Towards polyvalent ion batteries: a zinc-ion battery based on NASICON structured Na3 V2(PO4)3. Nano Energy 25, 211–217 (2016)
Wang, D., Wei, Q., Sheng, J., et al.: Flexible additive free H2V3O8 nanowire membrane as cathode for sodium ion batteries. Phys. Chem. Chem. Phys. 18, 12074–12079 (2016)
Mason, C.W., Lange, F.: Aqueous ion battery systems using sodium vanadium phosphate stabilized by titanium substitution. ECS Electrochem. Lett. 4, A79–A82 (2015)
He, P., Yan, M., Zhang, G., et al.: Layered VS2 nanosheet-based aqueous Zn ion battery cathode. Adv. Energy Mater. 7, 1601920 (2017)
Jo, J.H., Sun, Y.K., Myung, S.T.: Hollandite-type Al-doped VO1.52(OH)0.77 as a zinc ion insertion host material. J. Mater. Chem. A 5, 8367–8375 (2017)
Xu, H.T., Zhang, H., Liu, L., et al.: Fabricating hexagonal Al-doped LiCoO2 nanomeshes based on crystal-mismatch strategy for ultrafast lithium storage. ACS Appl. Mater. Interfaces 7, 20979–20986 (2015)
Reed, L.D., Menke, E.: The roles of V2O5 and stainless steel in rechargeable Al-ion batteries. J. Electrochem. Soc. 160, A915–A917 (2013)
Wang, H., Bai, Y., Chen, S., et al.: Binder-free V2O5 cathode for greener rechargeable aluminum battery. ACS Appl. Mater. Interfaces 7, 80–84 (2015)
Gu, S., Wang, H., Wu, C., et al.: Confirming reversible Al3+ storage mechanism through intercalation of Al3+ into V2O5 nanowires in a rechargeable aluminum battery. Energy Storage Mater. 6, 9–17 (2017)
Chiku, M., Takeda, H., Matsumura, S., et al.: Amorphous vanadium oxide/carbon composite positive electrode for rechargeable aluminum battery. ACS Appl. Mater. Interfaces 7, 24385–24389 (2015)
Endres, F.: Physical chemistry of ionic liquids. Phys. Chem. Chem. Phys. 12, 1648 (2010)
Koketsu, T., Ma, J., Morgan, B.J., et al.: Reversible magnesium and aluminium ions insertion in cation-deficient anatase TiO2. Nat. Mater. 16, 1142–1148 (2017)
Wang, H., Gu, S., Bai, Y., et al.: High-voltage and noncorrosive ionic liquid electrolyte used in rechargeable aluminum battery. ACS. Appl. Mater. Interfaces 8, 27444–27448 (2016)
Wang, H., Gu, S., Bai, Y., et al.: Anion-effects on electrochemical properties of ionic liquid electrolytes for rechargeable aluminum batteries. J. Mater. Chem. A 3, 22677–22686 (2015)
González, J.R., Nacimiento, F., Cabello, M., et al.: Reversible intercalation of aluminium into vanadium pentoxide xerogel for aqueous rechargeable batteries. RSC Adv. 6, 62157–62164 (2016)
Wang, W., Jiang, B., Xiong, W., et al.: A new cathode material for super-valent battery based on aluminium ion intercalation and deintercalation. Sci. Rep. 3, 3383 (2013)
Zhang, C., Song, H., Liu, C., et al.: Fast and reversible Li ion insertion in carbon-encapsulated Li3VO4 as anode for lithium-ion battery. Adv. Funct. Mater. 25, 3497–3504 (2015)
Li, Q., Wei, Q., Sheng, J., et al.: Mesoporous Li3VO4/C submicron-ellipsoids supported on reduced graphene oxide as practical anode for high-power lithium-ion batteries. Adv. Sci. 2, 1500284 (2015)
Jiang, J., Li, H., Huang, J., et al.: Investigation of the reversible intercalation/deintercalation of Al into the novel Li3VO4@C microsphere composite cathode material for aluminum-ion batteries. ACS. Appl. Mater. Interfaces 9, 28486–28494 (2017)
Nacimiento, F., Cabello, M., Alcántara, R., et al.: NASICON-type Na3V2(PO4)3 as a new positive electrode material for rechargeable aluminium battery. Electrochim. Acta 260, 798–804 (2017)
Sun, X.G., Zhang, Z., Guan, H.Y., et al.: A sodium-aluminum hybrid battery. J. Mater. Chem. A 5, 6589–6596 (2017)
Anasori, B., Lukatskaya, M.R., Gogotsi, Y.: 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2, 16098 (2017)
Vahidmohammadi, A., Hadjikhani, A., Shahbazmohamadi, S., et al.: Two-dimensional vanadium carbide (MXene) as a high capacity cathode material for rechargeable aluminum batteries. ACS Nano 11, 11135–11144 (2017)
Zhang, X., Wang, S., Tu, J., et al.: Flower-like vanadium sulfide/reduced graphene oxide composite: an energy storage material for aluminum-ion batteries. ChemSusChem 11, 709–715 (2018)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51602239, 51521001), the National Key Research and Development Program of China (2016YFA0202603, 2016YFA0202601), the National Basic Research Program of China (2013CB934103), the Programme of Introducing Talents of Discipline to Universities (B17034), the Hubei Provincial Natural Science Foundation of China (2016CFB267), the International Science and Technology Cooperation Program of China (2013DFA50840) and the Fundamental Research Funds for the Central Universities (WUT: 2017III009, 2017III005).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding authors
Rights and permissions
About this article
Cite this article
Tang, H., Peng, Z., Wu, L. et al. Vanadium-Based Cathode Materials for Rechargeable Multivalent Batteries: Challenges and Opportunities. Electrochem. Energ. Rev. 1, 169–199 (2018). https://doi.org/10.1007/s41918-018-0007-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41918-018-0007-y